| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
SHIP1/SH2 domain-containing inositol-5′-phosphatase 1 (IC50 = 2.5 μM)
3α-Aminocholestane (3AC) therapy substantially reduces OPM2 cell viability. When compared to OPM2 cells, RPMI8226 and U266 cells exhibit much lower sensitivity to 3α-Aminocholestane treatment; yet, viability is significantly reduced at doses of ≥12.5 μM. After being treated for 36 hours with 3α-Aminocholestane, the proportion of cells in the S phase is significantly decreased, and the number of cells in the G2/M phase increases. On the other hand, in the less proliferative RPMI8226 and U266 cells, treatment with 3α-Aminocholestane blocks cell cycle progression in the G0 /G1 phase and results in a lower percentage of cells progressing through the S phase[2]. |
|---|---|
| 体外研究 (In Vitro) |
3α-氨基胆甾烷 (3AC) 疗法显着降低 OPM2 细胞活力。与 OPM2 细胞相比,RPMI8226 和 U266 细胞对 3α-氨基胆甾烷治疗的敏感性要低得多;然而,剂量≥12.5 μM 时活力显着降低。 3α-氨基胆甾烷处理36小时后,S期细胞比例明显下降,G2/M期细胞数量增加。另一方面,在增殖能力较低的 RPMI8226 和 U266 细胞中,用 3α-氨基胆甾烷治疗可阻断 G0 /G1 期的细胞周期进程,并导致进入 S 期的细胞百分比较低[2]。
3α-氨基胆甾烷 (3AC) 选择性抑制 INPP5D (SHIP1) 的酶活性,IC50 约为 2.5 µmol/l,但对相关磷酸酶 INPP5L1 (SHIP2) 和 PTEN 的抑制活性不显著 (IC50 >20 µmol/l)。[1] 用 3α-氨基胆甾烷 (3AC) 处理患者来源的 Ph+ ALL 细胞可强烈诱导 SYK 的超活化(Y352位点磷酸化)。[1] 用 3α-氨基胆甾烷 (3AC) 处理患者来源的 TKI 耐药 Ph+ ALL 细胞可在四天内诱导细胞死亡。[1] 剂量反应分析显示,3α-氨基胆甾烷 (3AC) 对患者来源的 Ph+ ALL 细胞具有选择性毒性(IC50=2.8 µmol/l;n=5),而对成熟 B 细胞淋巴瘤细胞毒性较低(n=5)。[1] 用 SYK 抑制剂 PRT06207 预处理 Ph+ ALL 细胞,可在很大程度上保护细胞免受 3α-氨基胆甾烷 (3AC) 诱导的死亡,证明 Syk 的超活化是诱导细胞死亡所必需的。[1] 3α-氨基胆甾烷 (3AC) 在所有六例测试的、在 TKI 治疗下复发的 Ph+ ALL 病例(包括三例因 BCR-ABL1[T315I] 突变导致全局性 TKI 耐药的病例)中均诱导了大规模的细胞死亡(>95%)。相比之下,TKI 伊马替尼在 BCR-ABL1[T315I] 病例中无效。[1] |
| 体内研究 (In Vivo) |
在 OPM2 攻击后,发现 3α-氨基胆甾烷 (3AC) 会导致体内多发性骨髓瘤 (MM) 生长减少(通过血浆中游离人 Igλ 轻链的量来测量)。此外,通过人 HLA-ABC 标记检测,与载体对照相比,用 3-氨基胆甾烷处理的小鼠的外周血显示出较少的循环 OPM2 细胞。最值得注意的是,用 3α-氨基胆甾烷治疗的小鼠在肿瘤攻击后的存活率大大提高。当用3α-氨基胆甾烷治疗的小鼠对治疗没有反应时,发现MM肿瘤有SHIP2的过度表达,这与体外处理OPM2细胞时相似,这意味着SHIP2表达较高的肿瘤细胞可能会被SHIP1选择抑制[2]。
用 3α-氨基胆甾烷 (3AC) 治疗携带 TKI 耐药患者来源(BCR-ABL1[T315I])Ph+ ALL 细胞的 NOD/SCID 移植受体小鼠,能显著延长总生存期(P=0.0002,对数秩检验)并通过生物发光成像显示降低白血病负荷。[1] |
| 酶活实验 |
磷酸酶酶活性检测[2]
荧光偏振法如前所述。简而言之,重组SHIP1或SHIP2在潜在化学抑制剂存在的情况下与其底物PtdIns(3,4,5)P3混合。将反应产物与ptdins (3,4)P2检测蛋白和荧光PI(3,4)P2探针混合。新合成的ptdins (3,4)P2取代了检测蛋白,从而增强了混合物中未结合的荧光探针,并降低了平均极化单位。因此,确定的SHIP抑制剂(2-苯基苯并[h]喹啉-4-基)-[2]哌啶基-甲醇盐酸盐(1PIE), 1-[(氯苯基)甲基]-2-甲基-5-(甲基硫)- 1h -吲哚-3-乙胺盐酸盐(2PIQ)和(2-adamantan-1-基-6,8-二氯喹啉-4-基)-吡啶-2-甲醇盐酸盐(6PTQ)随后通过孔雀石绿法或荧光偏振法检测重组SHIP1或SHIP2对游离磷酸盐产生的抑制作用。为了证明SHIP1和SHIP2对其他磷酸酶的选择性,我们从OPM2细胞中免疫沉淀SHIP1和肌醇5-磷酸酶ocl。为此,OPM2细胞在ip裂解缓冲液(20 mmol/L Tris、150 mmol/L NaCl、1 mmol/L EDTA、1 mmol/L EGTA、1% Triton × 100、1 mmol/L苯基甲基磺酰氟和Halt蛋白酶抑制剂)中裂解,并使用小鼠IgG抗体免疫沉淀SHIP1或OCRL。用免疫沉淀(IP)裂解缓冲液洗涤4次,用tris缓冲盐水(TBS)/MgCl2 (10 mmol/L)洗涤1次,用TBS/MgCl2重悬。将SHIP抑制剂(200 μmol/L)加入微球5 min,免疫沉淀的SHIP1在100 μmol/L PtdIns(3,4,5)P3 存在下孵育,免疫沉淀的OCRL在100 μmol/L PtdIns(4,5)P2存在下孵育30 min。按照厂家说明加入孔雀石绿溶液,20 min后读板。3α-氨基胆甾(3AC)的鉴定见前文。 研究引用指出,3α-氨基胆甾烷 (3AC) 选择性抑制 INPP5D (SHIP1) 的酶活性,IC50 约为 2.5 µmol/l,但对相关磷酸酶 INPP5L1 (SHIP2) 和 PTEN 的抑制活性不显著 (IC50 >20 µmol/l)。所提供的文本中未详细描述酶活性测定(如激酶活性、SPR、ITC、HTRF)的具体实验方案。[1] |
| 细胞实验 |
细胞活力测定[2]
随着化合物浓度的增加,细胞被处理三次或更多次。根据制造商的说明,用细胞计数试剂盒测定细胞活力。用化合物处理细胞的OD除以对照细胞的OD,以未处理细胞的百分比表示细胞活力。结果用三个单独实验的平均值±标准误差表示。在PIP加回实验中,用10 μmol/L SHIP抑制剂处理MCF-7细胞2 h,洗净细胞,加入新鲜培养基。细胞在不含(0 μmol/L)或含有(10或20 μmol/L) PtdIns(3,4)P2-diC16 (P-3416)或PtdIns(3,5)P2-diC16 (P-3516)的条件下培养36 h,用Dojindo细胞计数试剂盒测定细胞活力。 细胞活力测定[1] 将10万个人ALL细胞以50 μl的培养基接种于96孔板的每孔中。将伊马替尼或其他抑制剂稀释后,在100 μl培养基中按指定浓度孵育。3 d后,用细胞计数试剂盒-8测定活细胞数。以载药处理细胞的基线值作为参考(设为100%)计算折叠变化。 流式细胞术[1] 流式细胞术中使用的抗体见补充表6。对于细胞周期分析,根据制造商的说明使用BrdU流式细胞术试剂盒或Click-iT EdU流式细胞术检测试剂盒。为了评估细胞内ROS水平,将ALL细胞与1 μM 5-(和6-)氯甲基-2 ',7 ' -二氯二氢荧光素(CM-H2DCFDA)在37℃下孵育7分钟,使染料被ROS氧化。用PBS洗涤后,将细胞在37°C的PBS中再孵育15分钟,使细胞内酯酶使氧化形式的CM-H2DCFDA完全去乙酰化。然后通过流式细胞术直接分析荧光水平,对活细胞进行门控。 Western blotting [1] 细胞缓冲液中添加蛋白酶抑制剂鸡尾酒和磷酸酶抑制剂鸡尾酒套装II,用于裂解细胞。每个样品10 μg的蛋白裂解物在微型预制凝胶上分离,并转移到硝化纤维素膜上。蛋白质检测采用一抗、碱性磷酸酶偶联二抗和化学发光底物。一抗的详细情况见补充表7。 小鼠细胞的集落形成试验[1] 本实验使用了10,000个bcr - abl1转化的ALL细胞或100,000个cml样细胞。将细胞重悬于小鼠MethoCult培养基中,并在直径3cm的培养皿上镀上一盘水以防止蒸发。7 ~ 14天后,计数菌落。 用于信号激活的 Western blot 分析:用 3α-氨基胆甾烷 (3AC)(10 µmol/l)处理患者来源的 Ph+ ALL 细胞指定时间点(0、4、8、16、60 分钟)。然后裂解细胞,蛋白质裂解物通过凝胶电泳分离,转膜,并使用针对 SYK (Y352)、SRC (Y416)、BTK (Y223) 和 PLCγ2 (Y1217) 磷酸化形式的抗体进行检测,以评估近端 pre-BCR 信号传导的超活化。[1] 用于细胞活力测定:将 100,000 个人类 ALL 细胞接种在 96 孔板中。将 3α-氨基胆甾烷 (3AC) 稀释并在指定浓度下孵育。3 天后,使用细胞计数试剂盒测定细胞活力。以载体处理细胞的基线值为参考计算倍数变化。[1] 为了测试 SYK 超活化在 3α-氨基胆甾烷 (3AC) 诱导死亡中的作用,在添加 3α-氨基胆甾烷 (3AC)(7.5 µmol/l)之前,用 SYK 抑制剂 PRT06207(2.5 µmol/l)预处理 ALL 细胞两天,并监测活力。[1] 用不同浓度的 3α-氨基胆甾烷 (3AC) 处理患者来源的 Ph+ ALL 细胞(n=5)和成熟 B 细胞淋巴瘤细胞(n=5),生成剂量反应曲线以确定 IC50 值。[1] |
| 动物实验 |
3α-Aminocholestane is suspended in a 0.3% Klucel/H2O solution at 11.46 mM and administered by intraperitoneal injection of 100 μL solution. NOD/SCID/γcIL2R (NSG) mice
OPM2 Tumor Challenge Studies [2]
NOD/SCID/γcIL2R (NSG) mice (The Jackson Laboratory, Bar Harbor, ME, USA) were injected intraperitoneally with 1 × 107 OPM2 cells and 6 h later received an initial injection of 3α-aminocholestane (3AC) or vehicle. 3α-aminocholestane (3AC) was suspended in a 0.3% Klucel/H2O solution at 11.46 mmol/L and administered by intraperitoneal injection of 100-μL solution. Vehicle-treated mice received 100-μL injection of 0.3% Klucel/H2O solution. The final concentration of 3α-aminocholestane (3AC) in the treated mice was 60 μmol/L. The mice were then treated with 3α-aminocholestane (3AC) or vehicle daily for the next 6 d and then twice per week in the remaining 15 wks of the survival study. In some instances, tumors from the vehicle- or 3α-aminocholestane (3AC) -treated hosts were excised and single-cell suspensions were made for Western blot analysis of SHIP2 expression after mice were deemed to be moribund and recommended for humane euthanasia by veterinary staff. Enzyme-Linked Immunosorbent Assay for Human Igλ Light Chain in Mouse Peripheral Blood [2] Mice were bled into a serum collection tube 4 wks after the OPM2 challenge, and serum was obtained after pelleting of blood cells at 5,000g for 5 min. Human Igλ light chain amounts were determined using an Ig light chain detection kit from Biovendor per the manufacturer’s instructions. Detection of Circulating OPM2 Cells in Mouse Blood [2] Mice were bled into a blood collection tube 4 wks after OPM2 challenge and red cells were lysed. White blood cells were incubated with anti-CD16/32 to block Fc receptor binding and then stained with antibodies against human HLA-ABC, clone W6/32. Samples were acquired on an LSRII cytometer (Becton Dickinson), and dead cells were excluded from the analysis after cytometer acquisition by exclusion of cells that stained positively for DAPI (di aminido phenyl indol). Patient-derived Ph+ ALL cells (e.g., BLQ5 line with BCR-ABL1[T315I] mutation) were transduced with a lentiviral vector encoding firefly luciferase. These cells were then injected into sublethally irradiated NOD/SCID mice via tail vein injection. [1] Mice carrying the leukemia xenografts were treated with either 3α-Aminocholestane (3AC) (50 mg/kg) or vehicle control via intraperitoneal (ip) injection daily. [1] Leukemia progression was monitored in vivo using bioluminescence imaging with an IVIS system. D-luciferin was injected intraperitoneally 15 minutes before imaging. [1] Overall survival of mice in the treatment and control groups was compared using Kaplan-Meier analysis. [1] |
| 参考文献 | |
| 其他信息 |
B cells are selected for an intermediate level of B-cell antigen receptor (BCR) signalling strength: attenuation below minimum (for example, non-functional BCR) or hyperactivation above maximum (for example, self-reactive BCR) thresholds of signalling strength causes negative selection. In ∼25% of cases, acute lymphoblastic leukaemia (ALL) cells carry the oncogenic BCR-ABL1 tyrosine kinase (Philadelphia chromosome positive), which mimics constitutively active pre-BCR signalling. Current therapeutic approaches are largely focused on the development of more potent tyrosine kinase inhibitors to suppress oncogenic signalling below a minimum threshold for survival. We tested the hypothesis that targeted hyperactivation--above a maximum threshold--will engage a deletional checkpoint for removal of self-reactive B cells and selectively kill ALL cells. Here we find, by testing various components of proximal pre-BCR signalling in mouse BCR-ABL1 cells, that an incremental increase of Syk tyrosine kinase activity was required and sufficient to induce cell death. Hyperactive Syk was functionally equivalent to acute activation of a self-reactive BCR on ALL cells. Despite oncogenic transformation, this basic mechanism of negative selection was still functional in ALL cells. Unlike normal pre-B cells, patient-derived ALL cells express the inhibitory receptors PECAM1, CD300A and LAIR1 at high levels. Genetic studies revealed that Pecam1, Cd300a and Lair1 are critical to calibrate oncogenic signalling strength through recruitment of the inhibitory phosphatases Ptpn6 (ref. 7) and Inpp5d (ref. 8). Using a novel small-molecule inhibitor of INPP5D (also known as SHIP1), we demonstrated that pharmacological hyperactivation of SYK and engagement of negative B-cell selection represents a promising new strategy to overcome drug resistance in human ALL.
\n\nA small molecule inhibitor against INPP5D, 3-α-aminocholestane, 3AC (Extended Data Fig. 10f) selectively inhibited enzymatic activity of INPP5D (SHIP1; IC50 ~2.5 μmol/l) but not related phosphatases INPP5L1 (SHIP2) and PTEN (IC50 >20 μmol/l). Treatment of patient-derived Ph+ ALL cells with 3AC induced strong hyperactivation of SYK (Fig. 4a). In patient-derived myeloid CML samples, baseline levels of Syk activity were very low and not responsive to 3AC treatment (Extended Data Fig. 10g). Biochemical characterization of 3AC-mediated inhibition of INPP5D in patient-derived Ph+ ALL cells revealed potent and transient hyperactivation of proximal pre-BCR signaling molecules (Fig. 4a). Treatment of patient-derived TKI-resistant Ph+ ALL cells with 3AC induced cell death within four days. Importantly, pre-treatment of Ph+ ALL cells with the SYK-inhibitor (PRT06207) largely protected Ph+ ALL cells against 3AC-induced cell death (Fig. 4b), demonstrating that hyperactivation of Syk is required for induction of cell death. Dose-response analyses revealed that 3AC is selectively toxic for patient-derived Ph+ ALL cells (IC50=2.8 μmol/l; n=5) compared to mature B cell lymphoma (n=5; Extended Data Fig. 10h). We next studied drug-responses in a panel of six cases of Ph+ ALL from patients who relapsed under TKI-therapy, including three cases with global TKI-resistance owing to the BCR-ABL1T315I mutation. As expected, treatment with the TKI Imatinib had no effect in BCR-ABL1T315I cases (Extended Data Fig. 10i). In contrast, 3AC induced massive cell death (>95%) in all six cases of Ph+ ALL regardless of BCR-ABL1 mutation status (Extended Data Fig. 10i). Likewise, treatment of NOD/SCID transplant recipient mice carrying TKI-resistant patient-derived (BCR-ABL1T315I) Ph+ ALL cells with 3AC significantly prolonged overall survival (P=0.0002, log rank test; Fig. 4c) and reduced leukemia burden (Fig. 4d). While further studies are needed to optimize pharmacological targeting of this pathway, these experiments identify transient hyperactivation of SYK and engagement of negative B cell selection as a powerful new strategy to overcome drug-resistance in Ph+ ALL. [1] \n\nMany tumors present with increased activation of the phosphatidylinositol 3-kinase (PI3K)-PtdIns(3,4,5)P(3)-protein kinase B (PKB/Akt) signaling pathway. It has long been thought that the lipid phosphatases SH2 domain-containing inositol-5'-phosphatase 1 (SHIP1) and SHIP2 act as tumor suppressors by counteracting with the survival signal induced by this pathway through hydrolysis or PtdIns(3,4,5)P(3) to PtdIns(3,4)P(2). However, a growing body of evidence suggests that PtdInd(3,4)P(2) is capable of, and essential for, Akt activation, thus suggesting a potential role for SHIP1/2 enzymes as proto-oncogenes. We recently described a novel SHIP1-selective chemical inhibitor (3α-aminocholestane [3AC]) that is capable of killing malignant hematologic cells. In this study, we further investigate the biochemical consequences of 3AC treatment in multiple myeloma (MM) and demonstrate that SHIP1 inhibition arrests MM cell lines in either G0/G1 or G2/M stages of the cell cycle, leading to caspase activation and apoptosis. In addition, we show that in vivo growth of MM cells is blocked by treatment of mice with the SHIP1 inhibitor 3AC. Furthermore, we identify three novel pan-SHIP1/2 inhibitors that efficiently kill MM cells through G2/M arrest, caspase activation and apoptosis induction. Interestingly, in SHIP2-expressing breast cancer cells that lack SHIP1 expression, pan-SHIP1/2 inhibition also reduces viable cell numbers, which can be rescued by addition of exogenous PtdIns(3,4)P(2). In conclusion, this study shows that inhibition of SHIP1 and SHIP2 may have broad clinical application in the treatment of multiple tumor types. \n\nAside from being a phosphatase, SHIP1 also functions to mask receptor tails to prevent recruitment of other signaling proteins, or as an adaptor protein for proteins such as Shc, DOK1 and Grb2, and as such has been proposed to reduce Ras signaling. Theoretically, it is possible that while blocking phosphatase activity with 3AC, these other functions of SHIP1 may not be affected. However, we observed a decrease in SHIP1 protein expression in MM cells upon prolonged treatment with 3AC, suggesting that these scaffolding functions may no longer play a role. It has recently been shown that SHIP-1 is ubiquitinated and targeted for proteasomal degradation upon its phosphorylation. However, we did not observe a difference in IGF-1–stimulated SHIP1 phosphorylation in MM cells after pretreatment with 3AC (unpublished observations, GM Fuhler). Hence, the reason for the proteasomal degradation of SHIP1 upon 3AC treatment remains unclear.[2] 3α-Aminocholestane (3AC) is a novel small molecule inhibitor of the phosphatase INPP5D (SHIP1). [1] It represents a pharmacological strategy to hyperactivate SYK kinase activity by inhibiting the negative regulator INPP5D. This engages a B-cell-intrinsic negative selection checkpoint against hyperactive tyrosine kinase signaling, leading to selective death of pre-B ALL cells. [1] This mechanism shows promise for overcoming drug resistance, particularly in TKI-resistant Ph+ ALL, including cases with the BCR-ABL1[T315I] mutation. [1] The vulnerability to INPP5D inhibition and subsequent SYK hyperactivation appears to be specific to B-lineage leukemia cells (like Ph+ ALL) and not observed in myeloid lineage leukemia (like CML) or normal pre-B cells under the conditions tested. [1] |
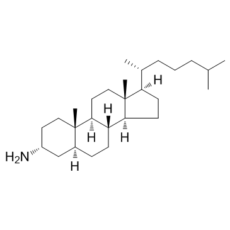
| 分子式 |
C27H49N
|
|
|---|---|---|
| 分子量 |
387.69
|
|
| 精确质量 |
387.386
|
|
| 元素分析 |
C, 83.65; H, 12.74; N, 3.61
|
|
| CAS号 |
2206-20-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5351709
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 熔点 |
104.5-105.5℃ (methanol )
|
|
| LogP |
9.1
|
|
| tPSA |
26
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
1
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
540
|
|
| 定义原子立体中心数目 |
9
|
|
| SMILES |
C[C@H](CCCC(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@@H]4[C@@]3(CC[C@H](C4)N)C)C
|
|
| InChi Key |
RJNGJYWAIUJHOJ-FBVYSKEZSA-N
|
|
| InChi Code |
InChI=1S/C27H49N/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h18-25H,6-17,28H2,1-5H3/t19-,20+,21-,22+,23-,24+,25+,26+,27-/m1/s1
|
|
| 化学名 |
(3R,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthren-3-amine
|
|
| 别名 |
3α-Aminocholestane; 3AC; 3-AC; 2206-20-4; 3alpha-Aminocholestane; 3; A-Aminocholestane; (3alpha,5alpha)-Cholestan-3-amine; (3R,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-amine; 3??-Aminocholestane; 3+/--Aminocholestane; 3 AC
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (8.38 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL的澄清EtOH储备液加入到400 μL PEG300中并混合均匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (8.38 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 EtOH 储备液添加到 900 μL 玉米油中并充分混合。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5794 mL | 12.8969 mL | 25.7938 mL | |
| 5 mM | 0.5159 mL | 2.5794 mL | 5.1588 mL | |
| 10 mM | 0.2579 mL | 1.2897 mL | 2.5794 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Small molecule inhibition of Inpp5d induces hyperactivation of Syk and triggers a deletional checkpoint in pre-B ALL cells.Nature.2015May 21;521(7552):357-61. |
|---|
SHIP1 inhibition reduces viable cell numbers and either G2/M or G0/G1 cell cycle arrest.Mol Med.2012 Feb 10;18:65-75. |
SHIP1 inhibition affects apoptosis induction differently in MM cell lines.Mol Med.2012 Feb 10;18:65-75. |