| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
PPARγ; NF-κB
|
|---|---|
| 体外研究 (In Vitro) |
4-O-Mmethyl honokiol 是从厚朴中分离出来的天然新木脂素,可作为 PPARγ 激动剂并降低 NF-κB 活性。 4-O-methylhonokio (20 μM) 可增强前列腺 PC-3 和 LNCap 细胞中的 PPARγ 表达、转录和 DNA 结合活性以及核转位。 4-O-Mmethyl honokiol (0-30 μM) 抑制 LNCaP 和 PC-3 癌细胞的生长,产生 G0/G1 期停滞并促进死亡,这种作用可以被 PPARγ 拮抗剂逆转。 4-O-methylhonokiol 可以降低 NF-κB 活性和癌细胞发育,但这种影响及其 PPARγ 的激活可以通过敲低 p21 来消除 [1]。 4-O-methylhonokiol(0.5、1 和 2 μM)可减少培养的星形胶质细胞以及培养的星形胶质细胞和小胶质细胞中 LPS 诱导的 NO、PGE2、ROS、TNF-α 和 IL-1β 的释放。神经胶质BV-2细胞中淀粉样蛋白的合成[2]。
在体外研究中,我们还发现4-O-甲基和厚朴酚抑制LPS刺激的培养星形胶质细胞中iNOS和COX-2的表达,以及活性氧、一氧化氮、前列腺素E2、肿瘤坏死因子-α和白细胞介素-1β的产生。4-O-甲基和厚朴酚还通过抑制IκB降解以及p50和p65易位到脑核和培养的星形胶质细胞中来抑制NF-κB的转录和DNA结合活性。与对神经炎症的抑制作用一致,4-O-甲基和厚朴酚抑制了LPS诱导的Aβ1-42生成、β和γ分泌酶活性、淀粉样前体蛋白(APP)、BACE1和C99的表达,以及脑、培养的星形胶质细胞和小胶质细胞BV-2细胞中星形胶质细胞的激活和神经元细胞死亡[2]。 |
| 体内研究 (In Vivo) |
在 SW620 和 PC3 异种移植模型中,4-O-甲基和厚朴酚(40 或 80 mg/kg,每天腹膜内给药,持续 4 周)减少了 SW620 和 PC3 肿瘤的生长。在肿瘤组织中,4-O-methylhonokio显着提高 p21 和 PPARγ 的表达 [1]。 4-O-甲基和厚朴酚(0.5 或 1 mg/kg/天,持续 3 周)可以阻止 LPS 诱导的小鼠 COX-2 和 iNOS 的产生,并显着减轻 LPS 诱导的记忆障碍。在用脂多糖 (LPS) 处理的小鼠大脑中,4-O-甲基和厚朴酚也表现出对 Aβ1-42 形成的抑制作用,并激活小胶质细胞和星形胶质细胞 [2]。
MH/4-O-甲基和厚朴酚在体内异种移植物模型中抑制SW620和PC3肿瘤的生长[1] 在PC3异种移植物研究中,MH/4-O-甲基和厚朴酚每天腹腔注射给肿瘤体积在100至300 mm3之间的小鼠,持续4周。在第28天,记录最终的肿瘤重量。在PC3肿瘤异种移植物中,用40和80 mg·kg-1的MH以及10 mg·kg−1的顺铂治疗的小鼠肿瘤体积分别为对照组的71.0%、57.7%和46.6%。在PC3肿瘤异种移植物中,用40和80 mg·kg-1的MH/4-O-甲基和厚朴酚和10 mg·kg−1的顺铂治疗的小鼠肿瘤重量分别为对照组的40.1%、30.9%和22.1%(图5A)。通过H&E对肿瘤切片的免疫组织化学分析,以及针对PCNA和Ki67染色的增殖抗原显示,40和80 mg·kg−1都能剂量依赖性地抑制肿瘤细胞的生长(图5B)。此外,MH治疗的肿瘤组织中p65和p50的核染色强度有降低的趋势(图5B)。此外,MH治疗的肿瘤中PPARγ对抗PPARγ的免疫反应也比未治疗的肿瘤组织更强烈。与体外抑制作用相似,MH增强了PPARγ的DNA结合活性,但抑制了肿瘤组织中NF-κB的活性(图5C)。MH增加了肿瘤组织中bax和切割caspases-3的表达,但降低了bcl-2的表达(图5D)。免疫组织化学分析还显示,与对照组相比,MH治疗的肿瘤组织中切割的胱天蛋白酶-3阳性细胞的表达显著增加。MH治疗的肿瘤组织中凋亡细胞死亡也显著增加(图5B)。此外,MH显著增加了肿瘤组织中p21和PPARγ的表达(图5B-D)。 |
| 细胞实验 |
细胞生长试验[1]
将细胞(每孔5×104个细胞)铺在24孔板上。使用排除性台盼蓝测定法,在用MH(0-30μM)处理0-72小时的细胞中评估4-O-甲基和厚朴酚/MH的细胞生长抑制作用。 萤光素酶活性的转染和测定[1] 将细胞(每孔1×105个细胞)铺在24孔板中,根据制造商的规范,使用OPTI-MEN中的质粒和脂质体PLUS的混合物,用pNF-κB-Luc质粒(5×NF-κB;Stratagene,La Jolla,CA,USA)或质粒pFA-GAL4-PPARγ瞬时转染。在TNF-α(10 ng·mL-1)不存在(用于PPARγ活性测定)或存在(用于NF-κB活性测定)的情况下,用4-O-甲基和厚朴酚MH处理转染细胞8小时。为了诱导NF-κB萤光素酶活性,我们用TNF-α(10-ng·mL−1)共处理细胞。使用萤光素酶测定试剂盒测量萤光素酶活性。 流式细胞术细胞周期分析[1] 亚融合细胞在培养基中用4-O-甲基和厚朴酚MH(0-30μM)处理0-72小时。分析方法如其他地方所述(Ban等人,2009a)。 下拉分析[1] 制备MH/4-O-甲基和厚朴酚珠缀合物,并如前所述进行下拉分析(Shim等人,2008)。MH与溴化氰(CNBr)活化的Sepharose 4B结合。简而言之,将MH(1 mg)溶解在500μL偶联缓冲液(0.1 M NaHCO3和0.5 M NaCl,pH 6.0)中。将溴化氰活化的琼脂糖4B溶胀,用1mM HCl洗涤,然后用偶联缓冲液洗涤。将溴化氰活化的Sepharose 4B珠加入含MH的偶联缓冲液中,在4°C下孵育24小时。用三个循环的交替pH洗涤缓冲液(缓冲液1,0.1 M醋酸盐和0.5 M NaCl,pH 4.0;缓冲液2,0.1 M Tris-HCl和0.5 M氯化钠,pH 8.0)洗涤MH偶联的Sepharrose 4B。然后用结合缓冲液(0.05 M Tris-HCl和0.15 M NaCl,pH 7.5)平衡MH偶联的珠粒。如前所述,在不存在MH的情况下制备对照非偶联CNBr-活化的Sepharose 4B珠。对于下拉测定,将来自PC-3前列腺癌症细胞的PPARγ蛋白或细胞裂解物与MH-Sephorse 4B珠在反应缓冲液(50mM Tris,pH 7.5,5mM EDTA,150mM NaCl,1mM二硫代苏糖醇,0.01%Nonidet P-40,2μg·mL−1 BSA,0.02mM PMSF,1×蛋白酶抑制剂)中孵育。用缓冲液(50 mM Tris,pH 7.5,5 mM EDTA,150 mM NaCl,1 mM二硫苏糖醇,0.01%Nonidet P-40,0.02 mM PMSF)洗涤珠子五次,用PPARγ抗体或细胞裂解物通过免疫印迹分析结合到珠子上的蛋白质。 培养的细胞同时用LPS(1μg/ml)和溶解在0.05%乙醇中的几种浓度(0.5、1、2μM)的4-O-甲基和厚朴酚处理,24小时后收获细胞。进行蛋白质印迹,测定Aβ水平和分泌酶活性。[2] 一氧化氮和PGE2测定[2] 星形胶质细胞在96孔板中生长,然后在不存在或存在不同浓度的4-O-甲基和厚朴酚的情况下与LPS(1μg/ml)一起孵育24小时。通过Griess反应评估上清液中亚硝酸盐的积累。将每50μl培养上清液与等体积的Griess试剂[0.1%N-(1-萘基)-乙二胺,1%磺胺在5%磷酸中]混合,在室温下孵育10分钟。在微孔板吸光度读数器中测量540 nm处的吸光度,并使用一系列已知浓度的亚硝酸钠作为标准。根据制造商的说明,在星形胶质细胞的培养上清液中,使用PGE2酶免疫测定(EIA)试剂盒(研发系统)测定PGE2浓度。 活性氧(ROS)的产生[2] 为了监测培养的星形胶质细胞中ROS的细胞内积累,使用了荧光探针2',7'-二氯荧光素二乙酸酯(DCF-DA)。在存在或不存在4-O-甲基和厚朴酚l(0.5、1、2μM)的情况下,用LPS(1μg/ml)处理24小时后,在含有145 mM NaCl、5 mM氯化钾(KCl)、1 mM氯化镁(MgCl2)、1 mmol氯化钙(CaCl2)、4 mM碳酸氢钠(NaHCO3)、5.5 mM葡萄糖、10 mM HEPES的改良Kreb缓冲液中洗涤细胞,pH 7.4。将细胞悬浮液转移到塑料管中。通过在黑暗中注射5μM DCF-DA开始测量。在37°C下孵育30分钟后,用荧光计在Ex=485和Em=538 nm下测定生成量。 |
| 动物实验 |
Antitumour activity study in in vivo xenograft animal model [1]
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). SW620 and PC3 cells were injected s.c. (1 × 107 cells in 0.1 mL PBS per animal) into the lower right flanks of mice. After 20 days, when the tumours had reached an average volume of 300–400 mm3 or about 50 mm3 (for prostate), the tumour-bearing nude mice were i.p. injected with MH/4-O-methylhonokio (40 and 80 mg·kg−1 dissolved in 0.1% DMSO) twice per week for 3 weeks. Cisplatin (10 mg·kg−1) was also i.p. injected once a week as a positive control. The group treated with 0.1% DMSO was designated as the control. The tumour volumes were measured with vernier calipers and calculated by the following formula: (A × B2)/2, where A is the larger and B is the smaller of the two dimensions. Dosage (0.5 and 1 mg/kg/day) of 4-O-methylhonokio in this study was used by referring to our previous studies. 4-O-methylhonokio (15 and 30 μg/mouse) was added to drinking water (5 ml of average water consumption of mouse per day) and mice were allowed access for 3 weeks ad libitum before induction of memory impairment as shown in Figure 1B.[2] Lipopolysaccharide-induced memory impairment mouse model [2] All mice were housed in a room that was automatically maintained at 21-25°C and relative humidity (45-65%) with a controlled light-dark cycle. Several researchers reported that repeated i.p. injection of LPS induced cognitive impairment like AD in mice. We therefore used this method as an AD mice model. The LPS (final concentration of 0.1 mg/ml) was dissolved, and aliquots in saline were stored at -20°C until use. The i.p. injection (250 μg/kg) of LPS or control (saline) was daily administered for 7 days. Subsequently, the behavioral tests of learning and memory capacity were assessed using two separate tests (water maze and passive avoidance test). One day interval was given between tests for adaptation of new circumstances as shown in Figure 1B. Water maze test [2] The water maze test is also a widely accepted method for memory test, and we performed this test as described by Morris et al. Maze testing was performed by the SMART-CS program and equipment. A circular plastic pool (height: 35 cm, diameter: 100 cm) was filled with milky water kept at 22-25°C. An escape platform (height: 14.5 cm, diameter: 4.5 cm) was submerged 0.5-1 cm below the surface of the water in position. On training trials, the mice were placed in a pool of water and allowed to remain on the platform for 10 s and were then returned to the home cage during the second-trial interval. The mice that did not find the platform within 60 s were placed on the platform for 10 s at the end of trial. They were allowed to swim until they sought the escape platform. These trials were performed in single platform and in three starting positions of rotational starting. Escape latency, escape distance, swimming speed and swimming pattern of each mouse was monitored by a camera above the center of the pool connected to a SMART-LD program. Probe test [2] A probe trial in order to assess memory consolidation was performed 24 h after the 5-day acquisition tests. In this trial, the platform was removed from the tank, and the mice were allowed to swim freely. For these tests, the percentage time in the target quadrant and target site crossings within 60 s was recorded. The time spent in the target quadrant is taken to indicate the degree of memory consolidation that has taken place after learning. The time spent in the target quadrant was used as a measure of spatial memory. Swimming pattern of each mouse was monitored by a camera above the center of the pool connected to a SMART-LD program described above. |
| 参考文献 |
|
| 其他信息 |
4-O-Methylhonokiol has been reported in Magnolia officinalis, Magnolia virginiana, and Magnolia obovata with data available.
Background and purpose: The effects of 4-O-methylhonokio (MH), a constituent of Magnolia officinalis, were investigated on human prostate cancer cells and its mechanism of action elucidated. Experimental approach: The anti-cancer effects of MH were examined in prostate cancer and normal cells. The effects were validated in vivo using a mouse xenograft model. Key results: MH increased the expression of PPARγ in prostate PC-3 and LNCap cells. The pull-down assay and molecular docking study indicated that MH directly binds to PPARγ. MH also increased transcriptional activity of PPARγ but decreased NF-κB activity. MH inhibited the growth of human prostate cancer cells, an effect attenuated by the PPARγ antagonist GW9662. MH induced apoptotic cell death and this was related to G(0) -G(1) phase cell cycle arrest. MH increased the expression of the cell cycle regulator p21, and apoptotic proteins, whereas it decreased phosphorylation of Rb and anti-apoptotic proteins. Transfection of PC3 cells with p21 siRNA or a p21 mutant plasmid on the cyclin D1/ cycline-dependent kinase 4 binding site abolished the effects of MH on cell growth, cell viability and related protein expression. In the animal studies, MH inhibited tumour growth, NF-κB activity and expression of anti-apoptotic proteins, whereas it increased the transcriptional activity and expression of PPARγ, and the expression of apoptotic proteins and p21 in tumour tissues. Conclusions and implication: MH inhibits growth of human prostate cancer cells through activation of PPARγ, suppression of NF-κB and arrest of the cell cycle. Thus, MH might be a useful tool for treatment of prostate cancer. [1] Background: Neuroinflammation is important in the pathogenesis and progression of Alzheimer disease (AD). Previously, we demonstrated that lipopolysaccharide (LPS)-induced neuroinflammation caused memory impairments. In the present study, we investigated the possible preventive effects of 4-O-methylhonokio, a constituent of Magnolia officinalis, on memory deficiency caused by LPS, along with the underlying mechanisms. Methods: We investigated whether 4-O-methylhonokiol (0.5 and 1 mg/kg in 0.05% ethanol) prevents memory dysfunction and amyloidogenesis on AD model mice by intraperitoneal LPS (250 μg/kg daily 7 times) injection. In addition, LPS-treated cultured astrocytes and microglial BV-2 cells were investigated for anti-neuroinflammatory and anti-amyloidogenic effect of 4-O-methylhonkiol (0.5, 1 and 2 μM). Results: Oral administration of 4-O-methylhonokiol ameliorated LPS-induced memory impairment in a dose-dependent manner. In addition, 4-O-methylhonokiol prevented the LPS-induced expression of inflammatory proteins; inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) as well as activation of astrocytes (expression of glial fibrillary acidic protein; GFAP) in the brain. In in vitro study, we also found that 4-O-methylhonokiol suppressed the expression of iNOS and COX-2 as well as the production of reactive oxygen species, nitric oxide, prostaglandin E2, tumor necrosis factor-α, and interleukin-1β in the LPS-stimulated cultured astrocytes. 4-O-methylhonokiol also inhibited transcriptional and DNA binding activity of NF-κB via inhibition of IκB degradation as well as p50 and p65 translocation into nucleus of the brain and cultured astrocytes. Consistent with the inhibitory effect on neuroinflammation, 4-O-methylhonokiol inhibited LPS-induced Aβ1-42 generation, β- and γ-secretase activities, and expression of amyloid precursor protein (APP), BACE1 and C99 as well as activation of astrocytes and neuronal cell death in the brain, in cultured astrocytes and in microglial BV-2 cells. Conclusion: These results suggest that 4-O-methylhonokiol inhibits LPS-induced amyloidogenesis via anti-inflammatory mechanisms. Thus, 4-O-methylhonokiol can be a useful agent against neuroinflammation-associated development or the progression of AD.[2] |
| 分子式 |
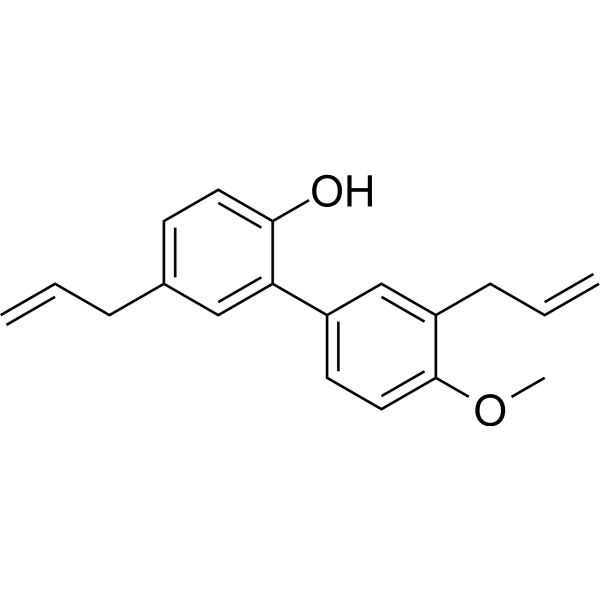
C19H20O2
|
|---|---|
| 分子量 |
280.3609
|
| 精确质量 |
280.146
|
| 元素分析 |
C, 81.40; H, 7.19; O, 11.41
|
| CAS号 |
68592-15-4
|
| PubChem CID |
155160
|
| 外观&性状 |
Orange to red viscous liquid
|
| 密度 |
1.054g/cm3
|
| 沸点 |
396.5ºC at 760 mmHg
|
| 闪点 |
176.2ºC
|
| 折射率 |
1.569
|
| LogP |
4.524
|
| tPSA |
29.46
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
339
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1C([H])([H])C([H])=C([H])[H])C1=C(C([H])=C([H])C(C([H])([H])C([H])=C([H])[H])=C1[H])O[H]
|
| InChi Key |
OQFHJKZVOALSPV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H20O2/c1-4-6-14-8-10-18(20)17(12-14)15-9-11-19(21-3)16(13-15)7-5-2/h4-5,8-13,20H,1-2,6-7H2,3H3
|
| 化学名 |
2-(4-methoxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol
|
| 别名 |
4-O-Methylhonokiol; 68592-15-4; 4-O-Methyl honokiol; METHYLHONOKIOL; 4-methoxyhonokiol;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~356.68 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.92 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.92 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5668 mL | 17.8342 mL | 35.6684 mL | |
| 5 mM | 0.7134 mL | 3.5668 mL | 7.1337 mL | |
| 10 mM | 0.3567 mL | 1.7834 mL | 3.5668 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。