| 规格 | 价格 | |
|---|---|---|
| 50mg | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
... In rats, radiolabelled cyprodinil administered by gavage as a single dose of 0.5 or 100 mg/kg bw, or as repeated doses of 0.5 mg/kg bw per day for 14 days, was rapidly absorbed from the gastrointestinal tract and excreted. Approximately 75% (range, 71-85%) of an orally administered dose was absorbed over 48 hr. At a dose of 0.5 and 100 mg/kg bw, two plasma level maxima of radioactivity were observed at approximately 0.5-1 hr and 8-12 hr, probably caused by reabsorption of material excreted in the bile. Approximately 92-97% of the administered dose was eliminated within 48 hr in the urine (48-68%), feces (29-47%), and bile (accounting for up to 35.4% of the dose in cannulated rats), with elimination being almost complete by day 7. Seven days after single or repeated oral administration at the lower dose, total tissue residues accounted for 0.15-0.60% of the administered dose. ... Excretion, distribution and metabolite profiles were essentially independent of dose, pretreatment and site of radiolabel, although there were some quantitative sex-dependent differences in urinary metabolites. After oral administration, CGA 219417 is rapidly absorbed and also rapidly and almost completely eliminated with urine and feces. ... Residues in tissues were generally low and there was no evidence for accumulation or retention of radioactivity. Metabolism / Metabolites In studies of metabolism in rats, ... cyprodinil was primarily metabolized by hydroxylation of the phenyl and pyrimidine rings and methyl group, and excreted mainly as glucuronide or sulfate conjugates in urine, feces and bile. Approximately 3-8% of the parent compound was detected in the feces. Excretion, distribution and metabolite profiles were essentially independent of dose, pretreatment and site of radiolabel, although there were some quantitative sex-dependent differences in urinary metabolites. The metabolic pathways are independent of sex, pre-treatment or dose level administered. In tomatoes, the metabolism of CGA 219417 proceeded mainly via hydroxylation of the 6-methyl group of the pyrimidine ring as well as hydroxylation of the phenyl & pyrimidine ring. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Cyprodinil is a fine beige powder. It is used as a foliar fungicide in cereals, grapes, pome fruits, stone fruits, strawberries, and vegetables and as a seed dressing on barley; it controls a wide range of pathogens, including Pseudocercosporella herpotrichoides, Erysiphe spp., Pyrenophora teres, Rhynchosporium secalis, and Septoria nodorum. HUMAN EXPOSURE AND TOXICITY: Cyprodinil acts as an aryl hydrocarbon receptor activator, a potential endocrine disrupter, and an extracellular signal-regulated kinase disrupter. Weak androgen receptor binding was shown for cyprodinil. Cyprodinil was genotoxic for HepG2 cells at concentrations 20 uM. ANIMAL STUDIES: In a 28 day gavage study in rats, the LOEL is 100 mg/kg bw/day for rats, based on increased liver weights and abnormalities in liver morphology. In a two-generation reproduction study in rats, the LOEL for maternal systemic toxicity is 4000 (about 326 mg/kg/day) based on lower body weights in the F0 females during the pre-mating period. The NOEL for maternal systemic toxicity is 1000 ppm (about 81 mg/kg/day). The LOEL for reproductive/developmental toxicity is 4000 ppm (about 326 mg/kg/day) based on decreased pup weights (F1 and F2). The NOEL for reproductive toxicity is 1000 ppm (about 81 mg/kg/day). In an 18-month carcinogenicity study in mice, the LOEL is 2000 ppm (males- 212.4 mg/kg/day) based on a dose-related increase in the incidence of focal and multifocal hyperplasia of the exocrine pancreas in males. The NOEL is 150 ppm (males- 16.1 mg/kg/day). This study was tested to adequate levels based on signs of toxicity in males at 2000 ppm and females at 5000 ppm. There was no indication of carcinogenic potential at any dose level. ECOTOXICITY STUDIES: In plants, cyprodinil promoted a copious increase in exudate secretion and caused the most severe collapse of stigmatic cells of all the fungicides evaluated. Toxicity Data LC50 (rat) > 1,200 mg/m3/4h Interactions ... A previous study identified the seven most common pesticide mixtures to which the French population was exposed through food consumption in 2006. The aim of this study was to investigate if the seven mixtures are potentially cytotoxic and genotoxic and if so, whether compounds in a same mixture have a combined effect. The cytotoxicity and genotoxicity of the seven mixtures were investigated with a new assay (gamma-H2AX) using four human cell lines (ACHN, SH-SY5Y, LS-174T, and HepG2). Mixtures were tested at equimolar concentrations and also at concentrations reflecting their actual proportion in the diet. Irrespective of the cell line tested, parallel cytotoxicity of the seven mixtures was observed. Only one mixture was genotoxic for the HepG2 cells at concentrations = 3 uM in equimolar proportion and at 30 uM in actual proportion. Caspase 3/7 activity, the comet assay, and reactive oxygen species production were also investigated using the same mixture and HepG2 cells. Our results suggest that pesticide metabolites from the mixture generated by HepG2 cells were responsible for the observed damage to DNA. Among the five compounds in the genotoxic mixture, only fludioxonil and cyprodinil were genotoxic for HepG2 cells alone at concentrations = 4 and 20 uM, respectively. Our data suggest a combined genotoxic effect of the mixture at low concentrations with a significantly higher effect of the mixture of pesticides than would be expected from the response to the individual compounds. Non-Human Toxicity Values LD50 Rat oral >2000 mg/kg LD50 Rat percutaneous >2000 mg/kg LC50 Rat inhalation >1200 mg/cu m/4 hr |
| 参考文献 | |
| 其他信息 |
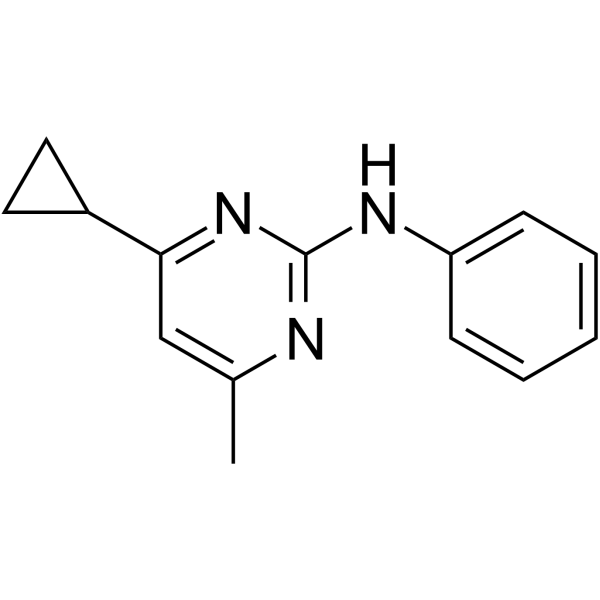
Cyprodinil is a member of the class of aminopyrimidine that is N-phenylpyrimidin-2-amine carrying additional cyclopropyl and methyl substituents at positions 4 and 6 respectively. A broad spectrum fungicide used to control a range of pathogens including Tapesia yallundae, Botrytis spp., Alternaria spp. and Rhynchospium secalis. Whilst it is a recognised irritant no serious human health concerns have been identified. It is moderately toxic to birds as well as most aquatic organisms and earthworms, but it is not considered toxic to honeybees. It has a role as an aryl hydrocarbon receptor agonist, an environmental contaminant, a xenobiotic and an antifungal agrochemical. It is an aminopyrimidine, a secondary amino compound, a member of cyclopropanes and an anilinopyrimidine fungicide.
Cyprodinil is a fungicide that acts by inhibition of germ tube elongation and hyphal mycelia. Cyprodinil is applied to the foliage of almonds, grapes, stone fruit crops, and pome fruit crops to control plant diseases. |
| 分子式 |
C14H15N3
|
|---|---|
| 分子量 |
225.29
|
| 精确质量 |
225.126
|
| CAS号 |
121552-61-2
|
| 相关CAS号 |
Cyprodinil-d5;1773496-67-5;Cyprodinil-13C6;1773496-63-1
|
| PubChem CID |
86367
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
406.0±48.0 °C at 760 mmHg
|
| 熔点 |
68 - 70ºC
|
| 闪点 |
199.3±29.6 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.659
|
| LogP |
4
|
| tPSA |
37.81
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
246
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HAORKNGNJCEJBX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H15N3/c1-10-9-13(11-7-8-11)17-14(15-10)16-12-5-3-2-4-6-12/h2-6,9,11H,7-8H2,1H3,(H,15,16,17)
|
| 化学名 |
4-cyclopropyl-6-methyl-N-phenylpyrimidin-2-amine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100 mg/mL (443.87 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (11.10 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (11.10 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.4387 mL | 22.1936 mL | 44.3872 mL | |
| 5 mM | 0.8877 mL | 4.4387 mL | 8.8774 mL | |
| 10 mM | 0.4439 mL | 2.2194 mL | 4.4387 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。