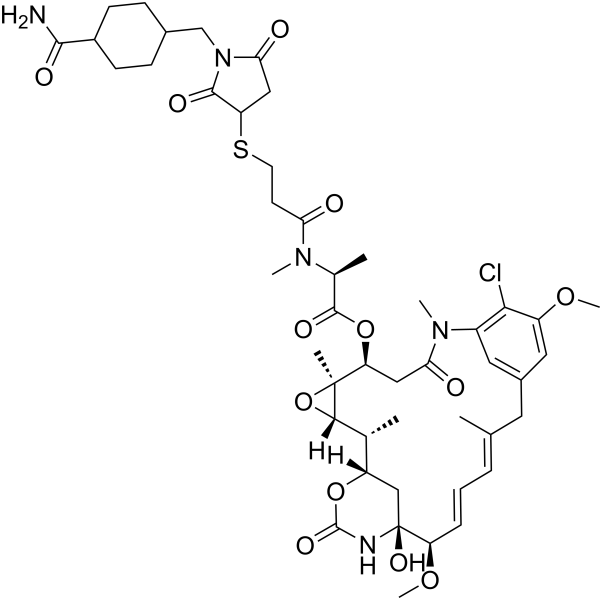
MCC-DM1 是一种生物活性分子-接头缀合物,用于 ADC 合成,类似于用于合成 Anti-CD22-MCC-DM1。
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
Assessing the Efficacy and Safety of Anti-HER2 Therapy in Nigerian Women With HER2+ Breast Cancer Before and After Surgery
CTID: NCT06348134
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-11-29
Testing the Use of Ado-Trastuzumab Emtansine Compared to the Usual Treatment (Chemotherapy With Docetaxel Plus Trastuzumab) for Recurrent, Metastatic, or Unresectable HER2-Positive Salivary Gland Cancer
CTID: NCT05408845
Phase: Phase 2 Status: Recruiting
Date: 2024-11-27
Tumor-Agnostic Precision Immuno-Oncology and Somatic Targeting Rational for You (TAPISTRY) Platform Study
CTID: NCT04589845
Phase: Phase 2 Status: Recruiting
Date: 2024-11-22
A Study of Trastuzumab Emtansine in Combination with Atezolizumab or Placebo As a Treatment for Participants with Human Epidermal Growth Factor 2 (HER2)-Positive and Programmed Death-ligand 1 (PD-L1)-Positive Locally Advanced (LABC) or Metastatic Breast Cancer (MBC)
CTID: NCT04740918
Phase: Phase 3 Status: Terminated
Date: 2024-11-19
Beamion BCGC-1: A Study to Find a Suitable Dose of Zongertinib in Combination With Trastuzumab Deruxtecan or With Trastuzumab Emtansine and to Test Whether it Helps People With Different Types of HER2+ Cancer That Has Spread
CTID: NCT06324357
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-11-18
View More
Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial)
CTID: NCT02465060
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-18
A Study to Evaluate the Safety and Effectiveness of Trastuzumab Emtansine (T-DM1) as Therapy in Chinese Participants With HER2 Positive Advanced Breast Cancer
CTID: NCT05945927
Phase: Status: Recruiting
Date: 2024-11-18
KPMNG Study of MOlecular Profiling Guided Therapy Based on Genomic Alterations in Advanced Solid Tumors II
CTID: NCT05525858
Phase: Status: Recruiting
Date: 2024-11-12
Testing Ado-Trastuzumab Emtansine as a Potential Targeted Treatment in Cancers With HER2 Genetic Changes (MATCH-Subprotocol Q)
CTID: NCT04439110
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-07
A Study To Evaluate the Efficacy and Safety Of Atezolizumab or Placebo in Combination With Neoadjuvant Doxorubicin + Cyclophosphamide Followed By Paclitaxel + Trastuzumab + Pertuzumab In Early Her2-Positive Breast Cancer
CTID: NCT03726879
Phase: Phase 3 Status: Completed
Date: 2024-11-05
A Study Evaluating the Efficacy and Safety of Adjuvant Atezolizumab or Placebo and Trastuzumab Emtansine for Participants With HER2-Positive Breast Cancer at High Risk of Recurrence Following Preoperative Therapy
CTID: NCT04873362
Phase: Phase 3 Status: Recruiting
Date: 2024-11-05
ELVN-002 in HER2 Mutant Non-Small Cell Lung Cancer
CTID: NCT05650879
Phase: Phase 1 Status: Recruiting
Date: 2024-10-29
A Study to Investigate Safety and Tolerability of TransCon IL-2 β/γ Alone or in Combination With Pembrolizumab and/or TransCon TLR7/8 Agonist or Other Anticancer Therapies in Adult Participants With Locally Advanced or Metastatic Solid Tumor Malignancies
CTID: NCT05081609
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-10-15
A Safety Extension Study of Trastuzumab Emtansine in Participants Previously Treated With Trastuzumab Emtansine Alone or in Combination With Other Anti-Cancer Therapy in One of the Parent Studies
CTID: NCT00781612
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-10-08
Feasibility of Chemotherapy De-escalation in Early-Stage HER2 Positive Breast Cancer
CTID: NCT04419181
Phase: Phase 2 Status: Suspended
Date: 2024-10-08
A Study to Evaluate Patient Preference for Home Administration of Fixed-Dose Combination of Pertuzumab and Trastuzumab for Subcutaneous Administration in Participants With Early or Locally Advanced/Inflammatory HER2-Positive Breast Cancer
CTID: NCT05415215
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-08
Phase Ib Dose-escalation Trial of Taselisib (GDC-0032) in Combination With Anti-HER2 Therapies in Participants With Advanced HER2+ Breast Cancer
CTID: NCT02390427
Phase: Phase 1 Status: Completed
Date: 2024-10-02
T-DM1 vs Paclitaxel/Trastuzumab for Breast (ATEMPT Trial)
CTID: NCT01853748
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-09-25
A Study Evaluating the Efficacy and Safety of Biomarker-Driven Therapies in Patients With Persistent or Recurrent Rare Epithelial Ovarian Tumors
CTID: NCT04931342
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-09-19
A Study of Trastuzumab Emtansine Versus Trastuzumab as Adjuvant Therapy in Patients With HER2-Positive Breast Cancer Who Have Residual Tumor in the Breast or Axillary Lymph Nodes Following Preoperative Therapy (KATHERINE)
CTID: NCT01772472
Phase: Phase 3 Status: Completed
Date: 2024-09-19
De-escalation Adjuvant Chemo in HER2+/ER-/node-neg Early BC Patients Who Achieved PCR After Neoadjuvant Chemo & Dual HER2 Blockade
CTID: NCT04675827
Phase: Phase 2 Status: Suspended
Date: 2024-09-19
HER2 Molecular Imaging with 89Zr-trastuzumab PET/CT As a Predictive Biomarker for Antibody-drug Conjugate Sequencing in Patients with Advanced HER2-positive Breast Cancer
CTID: NCT06595563
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-09-19
A Phase II Study of Tucatinib and Ado-trastuzumab Emtansine (T-DM1) in Patients with HER2-positive Metastatic Solid Tumors and Metastases to Brain (TUCATEMEB)
CTID: NCT05673928
Phase: Phase 2 Status: Recruiting
Date: 2024-09-19
T-DM1 and Tucatinib Compared With T-DM1 Alone in Preventing Relapses in People With High Risk HER2-Positive Breast Cancer, the CompassHER2 RD Trial
CTID: NCT04457596
Phase: Phase 3 Status: Recruiting
Date: 2024-08-02
A Study of Targeted Agents for Patients With Recurrent or Persistent Endometrial Cancer
CTID: NCT04486352
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2024-07-03
Secondary BRain Metastases Prevention After Isolated Intracranial Progression on Trastuzumab/Pertuzumab or T-DM1 in Patients With aDvanced Human Epidermal Growth Factor Receptor 2+ brEast Cancer With the Addition of Tucatinib
CTID: NCT05323955
Phase: Phase 2 Status: Recruiting
Date: 2024-06-06
DP303c Versus Trastuzumab Emtansine in in Patients With HER2-positive Advanced Breast Cancer
CTID: NCT06313086
Phase: Phase 3 Status: Recruiting
Date: 2024-05-10
CompassHER2-pCR: Decreasing Chemotherapy for Breast Cancer Patients After Pre-surgery Chemo and Targeted Therapy
CTID: NCT04266249
Phase: Phase 2 Status: Recruiting
Date: 2024-04-18
Trastuzumab Emtansine (T-DM1) in HER2-positive Breast Cancer Patients With Progressive Disease After TKIs or HP Therapy
CTID: NCT06125834
Phase: Phase 2 Status: Recruiting
Date: 2024-04-16
Safety of Continuing HER-2 Directed Therapy in Overt Left Ventricular Dysfunction
CTID: NCT04680442
Phase: Phase 2 Status: Recruiting
Date: 2024-03-15
Stereotactic Radiation Therapy for HE2-positive Oligometastatic Breast Cancer
CTID: NCT06299852
Phase: N/A Status: Recruiting
Date: 2024-03-08
A Study Evaluating Targeted Therapies in Participants Who Have Advanced Solid Tumors With Genomic Alterations or Protein Expression Patterns Predictive of Response
CTID: NCT04632992
Phase: Phase 2 Status: Completed
Date: 2024-03-07
A Study of Trastuzumab Emtansine (T-DM1) Plus Pertuzumab/Pertuzumab Placebo Versus Trastuzumab [Herceptin] Plus a Taxane in Participants With Metastatic Breast Cancer (MARIANNE)
CTID: NCT01120184
Phase: Phase 3 Status: Completed
Date: 2024-03-04
Serial Measurements of Molecular and Architectural Responses to Therapy (SMMART) PRIME Trial
CTID: NCT03878524
Phase: Phase 1 Status: Terminated
Date: 2024-03-04
SMMART Adaptive Clinical Treatment (ACT) Trial
CTID: NCT05238831
PhaseEarly Phase 1 Status: Withdrawn
Date: 2024-01-23
TPIV100 and Sargramostim for the Treatment of HER2 Positive, Stage II-III Breast Cancer in Patients With Residual Disease After Chemotherapy and Surgery
CTID: NCT04197687
Phase: Phase 2 Status: Recruiting
Date: 2023-12-18
Deciphering Antitumour Response and Resistance With INtratumour Heterogeneity
CTID: NCT02314481
Phase: Phase 2 Status: Active, not recruiting
Date: 2023-12-04
A Phase III, Active-Controlled Study of SHR-A1811 Versus Trastuzumab Emtansine (T-DM1) in HER2-Positive Primary Breast Cancer Participants With Residual Invasive Disease Following Neoadjuvant Therapy
CTID: NCT06126640
Phase: Phase 3 Status: Not yet recruiting
Date: 2023-11-13
The Rome Trial From Histology to Target: the Road to Personalize Target Therapy and Immunotherapy
CTID: NCT04591431
Phase: Phase 2 Status: Active, not recruiting
Date: 2023-10-03
Efficacy and Safety of Trastuzumab Emtansine in Chinese Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Locally Advanced or Metastatic Breast Cancer
CTID: NCT03084939
Phase: Phase 3 Status: Completed
Date: 2023-05-06
Copanlisib in Combination With T-DM1 in Pretreated Unresectable Locally Advanced or Metastatic HER2-positive Breast Cancer
CTID: NCT04042051
Phase: Phase 1 Status: Terminated
Date: 2023-04-06
A Study of MRG002 in the Treatment of Patients With HER2-positive Unresectable Locally Advanced or Metastatic Breast Cancer
CTID: NCT04924699
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2023-03-17
Observational Study of Effectiveness and Safety of Trastuzumab Emtansine (T-DM1) in HER2-positive Breast Cancer Patients With Residual Invasive Disease Following Neoadjuvant Chemotherapy and Anti-HER2 Target Therapy
CTID: NCT05754502
Phase: Status: Recruiting
Date: 2023-03-03
T-DM1 and Osimertinib Combination Treatment to Target HER2 Bypass Track Resistance in EGFR Mutation Positive NSCLC
CTID: NCT03784599
Phase: Phase 2 Status: Terminated
Date: 2022-11-10
ProTarget - A Danish Nationwide Clinical Trial on Targeted Cancer Treatment Based on Genomic Profiling
CTID: NCT04341181
Phase: Phase 2 Status: Recruiting
Date: 2022-10-26
Phase II Clinical Study of T-DM1 and Pyrotinib Maleate in Patients With HER2-positive Metastatic Breast Cancer Who Had Progressed on TKI Therapy
CTID: NCT05560308
Phase: N/A Status: Not yet recruiting
Date: 2022-10-03
A Study of Trastuzumab Emtansine (Kadcyla) Plus Pertuzumab (Perjeta) Following Anthracyclines in Comparison With Trastuzumab (Herceptin) Plus Pertuzumab and a Taxane Following Anthracyclines as Adjuvant Therapy in Participants With Operable HER2-Positive Primary Breast Cancer
CTID: NCT01966471
Phase: Phase 3 Status: Completed
Date: 2022-06-14
T-DM1 With or Without Abemaciclib for the Treatment of HER2-Positive Metastatic Breast Cancer
CTID: NCT04351230
Phase: Phase 2 Status: Withdrawn
Date: 2022-05-26
A Study of Trastuzumab Emtansine in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer Who Have Received Prior Anti-HER2 And Chemotherapy-based Treatment
CTID: NCT01702571
Phase: Phase 3 Status: Completed
Date: 2022-04-04
A Stud
A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED CLINICAL TRIAL TO EVALUATE THE EFFICACY AND SAFETY OF ADJUVANT ATEZOLIZUMAB OR PLACEBO AND TRASTUZUMAB EMTANSINE FOR HER2-POSITIVE BREAST CANCER AT HIGH RISK OF RECURRENCE FOLLOWING PREOPERATIVE THERAPY.
CTID: null
Phase: Phase 3 Status: Trial now transitioned, Ongoing
Date: 2021-03-01
A RANDOMIZED, MULTICENTER, DOUBLEBLIND, PLACEBO-CONTROLLED PHASE III STUDY OF THE EFFICACY AND SAFETY OF TRASTUZUMAB EMTANSINE IN COMBINATION WITH ATEZOLIZUMAB OR PLACEBO IN PATIENTS WITH HER2-POSITIVE AND PD-L1-POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER WHO HAVE RECEIVED PRIOR TRASTUZUMAB- (+/- PERTUZUMAB) AND TAXANE-BASED THERAPY (KATE3).
CTID: null
Phase: Phase 3 Status: Prematurely Ended, Completed
Date: 2021-01-27
A Phase 3, Multicenter, Randomized, Open-Label, Active-Controlled Study of Trastuzumab Deruxtecan (T-DXd) Versus Trastuzumab Emtansine (T-DM1) in Subjects with High-Risk HER2-Positive Primary Breast Cancer Who Have Residual Invasive Disease in Breast or Axillary Lymph Nodes Following Neoadjuvant Therapy
CTID: null
Phase: Phase 3 Status: Trial now transitioned, Ongoing
Date: 2021-01-21
A PHASE Ib/II, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY EVALUATING THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND EFFICACY OF VENETOCLAX IN COMBINATION WITH TRASTUZUMAB EMTANSINE IN PATIENTS WITH PREVIOUSLY TREATED HER2-POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER
CTID: null
Phase: Phase 1, Phase 2 Status: Prematurely Ended
Date: 2020-07-24
The ROME trial from histology to target: the road to personalize target therapy and immunotherapy
CTID: null
Phase: Phase 2 Status: Ongoing
Date: 2020-07-08
ProTarget
CTID: null
Phase: Phase 2 Status: Trial now transitioned
Date: 2020-04-28
A phase II trial of an individualized treatment strategy for patients with metastatic non-clear cell renal carcinoma
CTID: null
Phase: Phase 2 Status: Trial now transitioned
Date: 2019-11-20
EXPLORING OPTIMAL SEQUENCE TREATMENT IN HER2+ PERTUZUMAB PRE- TREATED ADVANCED BREAST CANCER PATIENTS. THE STEP TRIAL.
CTID: null
Phase: Phase 2 Status: Ongoing
Date: 2019-09-30
Atezolizumab, Pertuzumab and Trastuzumab with chemotherapy as neoadjuvant treatment of HER2 positive early high-risk and locally advanced breast cancer.
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2019-03-11
PREDIX II HER2. Improving pre-operative systemic therapy for human epidermal growth factor receptor 2 (HER2) amplified breast cancer
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2019-03-06
A Phase 3, multicenter, randomized, open-label, active-controlled study of trastuzumab deruxtecan (DS-8201a), an anti-HER2-antibody drug
CTID: null
Phase: Phase 3 Status: Trial now transitioned, GB - no longer in EU/EEA, Ongoing
Date: 2019-02-06
A phase III, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of Atezolizumab or placebo in combination with neoadjuvant doxorubicin + cyclophosphamide followed by paclitaxel + trastuzumab + pertuzumab in early HER2-positive breast cancer
CTID: null
Phase: Phase 3 Status: Temporarily Halted, Completed
Date: 2018-11-27
Multicentre, non-randomised, open-label, single agent phase II study to determine the clinical benefit of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer patients with brain metastasis
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2018-01-26
A RANDOMIZED, MULTICENTER, DOUBLE-BLIND, PLACEBO-CONTROLLED PHASE II STUDY OF THE EFFICACY AND SAFETY OF TRASTUZUMAB EMTANSINE IN COMBINATION WITH ATEZOLIZUMAB OR ATEZOLIZUMAB-PLACEBO IN PATIENTS WITH HER2-POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER WHO HAVE RECEIVED PRIOR TRASTUZUMAB AND TAXANE BASED THERAPY.
CTID: null
Phase: Phase 2 Status: Completed, Prematurely Ended
Date: 2016-10-21
PHASE II, EXPLORATORY, MULTICENTER, NON RANDOMIZED, SINGLE AGENT COHORT STUDY TO DETERMINE BEST TUMOR RESPONSE WITH TRASTUZUMAB EMTANSINE IN HER2 OVEREXPRESSING SOLID TUMORS.
CTID: null
Phase: Phase 2 Status: Completed
Date: 2016-09-22
Molecular-biological tumor profiling for drug treatment selection in patients with advanced and refractory carcinoma
CTID: null
Phase: Phase 2 Status: Completed
Date: 2015-05-04
A PHASE II, MULTICENTER, SINGLE-ARM STUDY OF TRASTUZUMAB EMTANSINE IN PATIENTS WITH HER2 IHC-POSITIVE, LOCALLY ADVANCED OR METASTATIC NON−SMALL CELL LUNG CANCER WHO HAVE RECEIVED AT LEAST ONE PRIOR CHEMOTHERAPY REGIMEN
CTID: null
Phase: Phase 2 Status: Completed
Date: 2014-12-01
PREDIX HER2 - Neoadjuvant response-guided treatment of HER2 positive breast cancer. Part of a platform of translational phase II trials based on molecular subtypes
CTID: null
Phase: Phase 2 Status: Trial now transitioned
Date: 2014-11-12
A Phase II, Randomized Study of T DM1 versus T DM1 plus short induction with docetaxel in first line treatment for locally advanced or metastatic HER2+ breast cancer.
CTID: null
Phase: Phase 2 Status: Ongoing
Date: 2014-10-20
A RANDOMIZED, MULTICENTER, OPEN-LABEL, TWO-ARM, PHASE III NEOADJUVANT STUDY EVALUATING TRASTUZUMAB EMTANSINE PLUS PERTUZUMAB COMPARED WITH CHEMOTHERAPY PLUS TRASTUZUMAB AND PERTUZUMAB FOR PATIENTS WITH HER2-POSITIVE BREAST CANCER.
CTID: null
Phase: Phase 3 Status: Completed
Date: 2014-08-11
A RANDOMIZED, MULTICENTER, OPEN-LABEL, PHASE III TRIAL COMPARING TRASTUZUMAB PLUS PERTUZUMAB PLUS A TAXANE FOLLOWING ANTHRACYCLINES VERSUS TRASTUZUMAB EMTANSINE PLUS PERTUZUMAB FOLLOWING ANTHRACYCLINES AS ADJUVANT THERAPY IN PATIENTS WITH OPERABLE HER2 POSITIVE PRIMARY BREAST CANCER.
CTID: null
Phase: Phase 3 Status: Temporarily Halted, GB - no longer in EU/EEA, Prematurely Ended, Completed
Date: 2014-01-13
Pertuzumab + trastuzumab (PH) versus PH plus metronomic chemotherapy (PHM) in the elderly HER2+ metastatic breast cancer population who may continue on T-DM1 alone following disease progression while on PH / PHM: an open-label multicenter randomized phase II selection trial of the EORTC Elderly Task Force and Breast Cancer Group
CTID: null
Phase: Phase 2 Status: GB - no longer in EU/EEA, Completed
Date: 2013-06-11
A RANDOMIZED, MULTICENTER, OPEN LABEL PHASE III STUDY TO EVALUATE THE EFFICACY AND SAFETY OF TRASTUZUMAB EMTANSINE VERSUS TRASTUZUMAB AS ADJUVANT THERAPY FOR PATIENTS WITH HER2-POSITIVE PRIMARY BREAST CANCER WHO HAVE RESIDUAL TUMOR PRESENT PATHOLOGICALLY IN THE BREAST OR AXILLARY LYMPH NODES FOLLOWING PREOPERATIVE THERAPY.
CTID: null
Phase: Phase 3 Status: Ongoing, GB - no longer in EU/EEA, Prematurely Ended, Completed
Date: 2013-04-09
A randomized phase II trial of pertuzumab in combination with trastuzumab with or without chemotherapy, both followed by T-DM1 in case of progression, in patients with HER2-positive metastatic breast cancer
CTID: null
Phase: Phase 2 Status: Completed
Date: 2013-02-04
A randomized, multicenter, adaptive phase II/III study to evaluate the efficacy and safety of trastuzumab emtansine (T-DM1) versus taxane (docetaxel or paclitaxel) in patients with previously treated locally advanced or metastatic HER2-positive gastric cancer, including adenocarcinoma of the gastroesophageal junction.
CTID: null
Phase: Phase 2, Phase 3 Status: Completed
Date: 2012-11-23
A two-cohort, open-label, multicenter, study of trastuzumab emtansine (T-DM1) in HER2-positive locally advanced or metastatic breast cancer patients who have received prior anti-HER2 and chemotherapy-based treatment
CTID: null
Phase: Phase 3 Status: Completed
Date: 2012-11-22
Phase I followed by phase II study of the combination of trastuzumab emtansine (T-DM1) and capecitabine in HER2-positive metastatic breast cancer and HER2-positive locally advanced or metastatic gastric cancer patients
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2012-08-22
Adjuvant Dynamic marker-Adjusted Personalized Therapy trial optimizing risk assessment and therapy response prediction in early breast cancer
CTID: null
Phase: Phase 2, Phase 3 Status: Ongoing
Date: 2012-03-29
A phase II prospective imaging study evaluating the utility of pre-treatment zirconium-89 labelled trastuzumab PET/CT and an early FDG-PET/CT response to identify patients with advanced HER-2 positive breast cancer unlikely to benefit from a novel anti-HER2 therapy: T-DM1
CTID: null
Phase: Phase 2 Status: Completed
Date: 2012-03-05
A Phase III randomized, multicenter, two-arm, open-label trial to evaluate the efficacy of trastuzumab emtansine compared with treatment of physician’s choice in patients with HER2-positive metastatic breast cancer who have received at least two prior regimens of HER2-directed therapy.
CTID: null
Phase: Phase 3 Status: Completed
Date: 2011-12-02
An open-label, multicenter extension study of trastuzumab emtansine administered as a single agent or in combination with other anti-cancer therapies in patients previously enrolled in a Genentech and /or F. Hoffmann-La Roche Ltd. - sponsored trastuzumab emtansine study.
CTID: null
Phase: Phase 2 Status: Trial now transitioned, Completed
Date: 2011-04-29
ESTUDIO FASE II MULTINACIONAL, MULTICÉNTRICO,PARA EVALUAR LA SEGURIDAD CLÍNICA Y VIABILIDAD DE LA ADMINISTRACIÓN DE T-DM1 DE FORMA SECUENCIAL CON UN RÉGIMEN DE QUIMIOTERAPIA BASADO EN ANTRACICLINAS, PARA EL TRATAMIENTO ADYUVANTE O NEOADYUVANTE DE PACIENTES CON CÁNCER DE MAMA HER2 POSITIVO PRECOZ
CTID: null
Phase: Phase 2 Status: Completed
Date: 2010-10-18
A randomized, 3 arm, multicentre, phase III study to evaluate the efficacy and the safety of T-DM1 combined with pertuzumab or T-DM1 combined with pertuzumab-placebo (blinded for pertuzumab), versus the combination of trastuzumab plus taxane, as first line treatment in HER2- positive progressive or recurrent locally advanced or metastatic breast cancer (MBC).
CTID: null
Phase: Phase 3 Status: Completed
Date: 2010-04-22
An open-label, multi-center study of the safety and tolerability of the combination of Trastuzumab-MCC-DM1 (T-DM1) with docetaxel, and potentially pertuzumab, for treatment for patients with advanced breast cancer.
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2009-08-24
A Phase Ib/II, open-label study of the safety, tolerability, and efficacy of trastuzumab-MCC-DM1 in combination with pertuzumab administered intravenously to patients with HER2-positive locally advanced or metastatic breast cancer who have progressed while receiving prior therapy
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2009-04-27
A randomized, multicenter, phase III open-label study of the efficacy and safety of trastuzumab-MCC-DM1 vs. capecitabine + lapatinib in patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy.
CTID: null
Phase: Phase 3 Status: Completed
Date: 2009-03-18
A randomized, multicenter, Phase II study of the efficacy and safety of trastuzumab-MCC-DM1 vs. trastuzumab (Herceptin®) and docetaxel (Taxotere®) in patients with metastatic HER2-positive breast cancer who have not received prior chemotherapy for metastatic disease
CTID: null
Phase: Phase 2 Status: Completed
Date: 2009-03-02