| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
对于治疗过敏性鼻炎和慢性荨麻疹,阿瓦斯汀(通常 8 毫克,每日 3 次)是一种有效且耐受性良好的抗组胺药。使用阿瓦斯汀治疗季节性过敏性鼻炎比使用安慰剂更成功,并且作用与氯马斯汀或特非那定相似。阿克伐斯汀的作用类似于常用剂量的氯马斯汀、羟嗪、扑尔敏、赛庚啶或特非那定,用于治疗由组胺引起的皮肤病。它也比安慰剂更有效。与氯马斯汀相比,阿瓦斯汀的困倦程度较低,其副作用与特非那定或安慰剂相似[1]。 4 毫克和 8 毫克剂量的阿瓦斯汀显着减轻了季节性过敏性鼻炎的症状,包括流鼻涕、打喷嚏的评分和总体评分。此外,8 毫克阿伐斯汀可降低流泪和喉咙沙哑的症状评分。在治疗季节性过敏性鼻炎时,阿瓦斯汀耐受性良好且效果良好[2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Acrivastine was absorbed rapidly from the combination capsule following oral administration and was as bioavailable as a solution of acrivastine. After administration of SEMPREX-D Capsules, maximum plasma acrivastine concentrations were achieved at 1.14 ± 0.23 hour. A mass balance study in 7 healthy volunteers showed that acrivastine is primarily eliminated by the kidneys. Over a 72-hour collection period, about 84% of the administered total radioactivity was recovered in urine and about 13% in feces, for a combined recovery of about 97%. 0.46 ± 0.05 L/kg 2.9 ± 0.7 mL/min/kg Biological Half-Life The mean terminal half-life for acrivastine was 1.9 ± 0.3 hours following single oral doses and increased to 3.5 ± 1.9 hours at steady state. The terminal half-life for the propionic acid metabolite was 3.8 ± 1.4 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Acrivastine has not been linked to liver enzyme elevations or to instances of clinically apparent liver injury. Its relative safety may relate to its rapid metabolism and use in low dosages. Likelihood score: E (unlikely cause of clinically apparent liver injury). References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines. Drug Class: Antihistamines Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Small occasional doses of acrivastine would not be expected to cause any adverse effects in breastfed infants. Larger doses or more prolonged use may cause drowsiness and other effects in the infant or decrease the milk supply, particularly in combination with a sympathomimetic such as pseudoephedrine or before lactation is well established. The nonsedating antihistamines are preferred alternatives. ◉ Effects in Breastfed Infants Relevant published information on acrivastine was not found as of the revision date. In one telephone follow-up study, mothers reported irritability and colicky symptoms 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. Whether lower oral doses of antihistamines have the same effect on serum prolactin or whether the effects on prolactin have any consequences on breastfeeding success have not been studied. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Acrivastine binding to human plasma proteins was 50 ± 2.0%. |
| 参考文献 |
|
| 其他信息 |
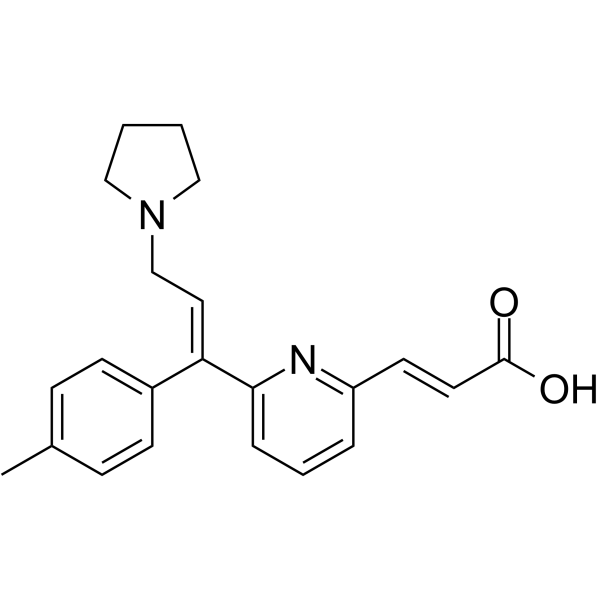
Acrivastine is a member of the class of pyridines that is (pyridin-2-yl)acrylic acid substituted at position 6 by a [(1E)-1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl group. It is a non-sedating antihistamine used for treatment of hayfever, urticaria, and rhinitis. It has a role as a H1-receptor antagonist. It is an alpha,beta-unsaturated monocarboxylic acid, a member of pyridines, a N-alkylpyrrolidine and an olefinic compound.
Acrivastine is a triprolidine analog antihistamine indicated for the treatment of allergies and hay fever. As an H1 receptor antagonist, it functions by blocking the action of histamine at this receptor thereby preventing the symptoms associated with histamine release such as pruritis, vasodilation, hypotension, edema, bronchoconstriction, and tachycardia. Acrivastine is currently available in combination with pseudoephedrine as the FDA-approved product Semprex-D. Acrivastine is a Histamine-1 Receptor Antagonist. The mechanism of action of acrivastine is as a Histamine H1 Receptor Antagonist. Acrivastine is a second generation antihistamine that is used for the treatment of allergic rhinitis. Acrivastine has not been linked to instances of clinically apparent acute liver injury. Acrivastine is a synthetic alkylamine with non-sedative antihistaminergic activity. Acrivastine competitively blocks the histamine H1 receptor and limits the typical allergic and anaphylactic responses, including bronchoconstriction, vasodilation, increased capillary permeability, and spasmodic contraction of gastrointestinal smooth muscle, caused by actions of histamine on bronchial, and gastrointestinal smooth muscles, and on capillaries. This drug also prevents histamine-induced pain and itching of the skin and mucous membranes. (NCI05) Drug Indication For the relief of symptoms associated with seasonal allergic rhinitis such as sneezing, rhinorrhea, pruritus, lacrimation, and nasal congestion. FDA Label |
| 分子式 |
C22H24N2O2
|
|---|---|
| 分子量 |
348.4382
|
| 精确质量 |
348.183
|
| CAS号 |
87848-99-5
|
| 相关CAS号 |
Acrivastine-d7;172165-56-9
|
| PubChem CID |
5284514
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
555.1±50.0 °C at 760 mmHg
|
| 熔点 |
55.5-59.5 °C(lit.)
|
| 闪点 |
289.5±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.627
|
| LogP |
4.55
|
| tPSA |
53.43
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
514
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C(/C1C=CC(C)=CC=1)(\C1C=CC=C(/C=C/C(=O)O)N=1)=C/CN1CCCC1
|
| InChi Key |
PWACSDKDOHSSQD-IUTFFREVSA-N
|
| InChi Code |
InChI=1S/C22H24N2O2/c1-17-7-9-18(10-8-17)20(13-16-24-14-2-3-15-24)21-6-4-5-19(23-21)11-12-22(25)26/h4-13H,2-3,14-16H2,1H3,(H,25,26)/b12-11+,20-13+
|
| 化学名 |
(E)-3-[6-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridin-2-yl]prop-2-enoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~143.50 mM)
H2O : ~1 mg/mL (~2.87 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8699 mL | 14.3497 mL | 28.6993 mL | |
| 5 mM | 0.5740 mL | 2.8699 mL | 5.7399 mL | |
| 10 mM | 0.2870 mL | 1.4350 mL | 2.8699 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01732510 | TERMINATEDWITH RESULTS | Drug: MK-8226 Drug: Placebo |
Atopic Dermatitis | Merck Sharp & Dohme LLC | 2012-12-21 | Phase 1 |