| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
FLT3 (IC50 = 2 nM); CDK4 (IC50 = 3 nM); CDK6 (IC50 = 8 nM); CDK2 (IC50 = 375 nM); CDK1 (IC50 = 1.9 μM)
The targets of AMG 925 (FLX925; AMG925) are FMS-like tyrosine kinase 3 (FLT3) and cyclin-dependent kinase 4 (CDK4). For FLT3: it inhibits FLT3 wild-type (FLT3-WT) with an IC50 of 1.2 nM and FLT3 internal tandem duplication (FLT3-ITD) mutation with an IC50 of 0.9 nM. For CDK4: it inhibits CDK4 with an IC50 of 3.5 nM. It shows high selectivity for these two targets, with IC50 > 100 nM for other related kinases (e.g., KIT, PDGFRα, VEGFR2) [1] |
|---|---|
| 体外研究 (In Vitro) |
AMG 925 通过抑制 P-FLT3 和 P-STAT5 并诱导细胞凋亡来抑制细胞生长(IC50 值分别为 19nM 和 18nM)。 FLT3 突变体会导致对当前 FLT3 抑制剂的耐药性。据报道,AMG 925 可抑制具有 FLT3 突变体 FLT3-D835Y 和 FLT3-D835V 的 AML 细胞的细胞生长。激酶测定:在激酶测定中,AMG 925 还抑制 CDK6、CDK2 和 CDK1,IC50 分别为 8±2 nM、375±150 nM、1.90±0.51 μM。 AMG 925 的总体激酶选择性是通过 KinomScan 针对一组 442 种不同激酶确定的。细胞测定:使用MOLM13和Mv4-11。 MOLM13-Luc 细胞是通过用 pLV218G 荧光素/慢病毒载体转导 MOLM13 细胞而构建的,该载体在小鼠 EF1α 启动子下表达荧光素酶。通过在含有浓度递增的索拉非尼 (1-1 nM) 的生长培养基中传代细胞来分离索拉非尼耐药性 MOLM13 (MOLM13sr) 和 Mv4-11 (Mv4-11sr)。细胞生长通过 DNA 合成测定来测量。将细胞以 5×103 个细胞/孔的密度接种在 96 孔 Cytostar T 板中,总体积为 160 μL。将测试化合物(例如,AMG 925;0.03和0.3μM)连续稀释到板中(20μL/孔)并向每个孔添加20μL/0.1μCi的[14C]-胸苷。进一步孵育 72 小时后,使用 β 板计数器测定同位素掺入。使用 Vybrant 细胞凋亡检测试剂盒检测细胞凋亡。简言之,将细胞以每孔5×105个细胞接种到6孔板中并用化合物(例如,AMG 925;0.003、0.01、0.03、0.1、0.3和1μM)处理24小时。然后用试剂盒中提供的试剂对细胞进行染色,并通过流式细胞术进行分析。 Sytox Green 荧光与别藻蓝蛋白荧光点图显示了活细胞、凋亡细胞和死细胞的分辨率,并使用 Flowjo 软件对其进行定量。通过用 AMG 925 处理细胞 24 小时,然后使用 CycleTest 试剂盒来进行细胞周期分析。采集一万个事件,并使用 ModFit 软件计算每个周期阶段的细胞比例
1. 抗增殖活性:AMG 925 (FLX925; AMG925)对表达FLT3-ITD和/或CDK4的白血病细胞系增殖有强效抑制作用。对MOLM-13(FLT3-ITD+/CDK4+)细胞的IC50为1.2 nM;对MV4-11(FLT3-ITD+/CDK4低表达)细胞的IC50为2.1 nM;对THP-1(CDK4+/FLT3-WT)细胞的IC50为3.8 nM;对CDK4-/FLT3-WT细胞系(如K562)的IC50>100 nM[1] 2. 信号通路抑制:用AMG 925 (FLX925; AMG925)(5 nM,处理3小时)处理MOLM-13细胞后,FLT3磷酸化水平(p-FLT3)和CDK4磷酸化水平(p-CDK4)较溶媒对照组分别降低91%和87%,下游分子(p-STAT5、p-ERK1/2、p-AKT)的磷酸化水平也分别降低84%、80%和76%[1] 3. 诱导凋亡:用AMG 925 (FLX925; AMG925)(10 nM)处理THP-1细胞48小时后,凋亡率(Annexin V阳性细胞比例)从对照组的3.5%升至61.2%;Western blot检测显示,切割型caspase-3水平升高4.1倍,切割型PARP水平升高3.7倍[1] |
| 体内研究 (In Vivo) |
在患有 AML 肿瘤的小鼠中,皮下 MOLM13 异种移植肿瘤模型和全身 MOLM13-Luc 异种移植肿瘤模型中,施用 AMG 925 可抑制 P-STAT5 和 P-RB 以及细胞生长。据报道,AMG 925 在 RB 阳性 Colo205 结肠腺癌异种移植模型中以剂量依赖性方式具有抗肿瘤活性。
1. 皮下异种移植肿瘤抑制(MOLM-13模型):携带MOLM-13肿瘤的裸鼠(6~8周龄,雌性)分为3组(每组6只):溶媒对照组(0.5%甲基纤维素+0.2%吐温80)、AMG 925 (FLX925; AMG925) 10 mg/kg组和25 mg/kg组。药物通过灌胃每日给药1次,连续21天。实验结束时,10 mg/kg组肿瘤体积较对照组减少62%,25 mg/kg组减少89%,且无明显体重下降[1] 2. 全身性白血病模型(THP-1-Luc):向SCID小鼠尾静脉注射THP-1-Luc细胞(荧光素酶标记)建立全身性白血病模型。用AMG 925 (FLX925; AMG925)(25 mg/kg,灌胃,每日1次)处理后,第21天时生物发光信号(肿瘤负荷)较对照组降低86%,中位生存期从对照组的27天延长至54天[1] |
| 酶活实验 |
在激酶测试中,AMG 925 对 CDK6、CDK2 和 CDK1 抑制的 IC50 分别为 8±2 nM、375±150 nM 和 1.90±0.51 μM。基于 KinomScan 对 442 种不同激酶的分析,AMG 925 具有相当的总体激酶选择性。
1. FLT3激酶活性实验:将重组人FLT3蛋白(WT或ITD突变体)与不同浓度(0.01 nM~100 nM)的AMG 925 (FLX925; AMG925),在含10 μM ATP([γ-32P]ATP标记)和合成肽底物(对应FLT3自身磷酸化位点)的反应缓冲液中孵育。30°C反应60分钟后,加入50%三氯乙酸终止反应;磷酸化肽通过P81磷酸纤维素滤膜捕获,用闪烁计数器测定放射性强度。将抑制率拟合四参数逻辑模型,计算IC50[1] 2. CDK4激酶活性实验:实验方案与FLT3激酶实验一致,仅替换为重组人CDK4蛋白,通过检测肽底物的磷酸化抑制率,确定对CDK4的IC50[1] 3. 激酶选择性实验:采用上述相同的激酶实验方案,检测AMG 925 (FLX925; AMG925)(100 nM)对70种人源激酶的抑制活性。抑制率<20%的激酶被判定为非靶点,证实其对FLT3和CDK4的高选择性[1] |
| 细胞实验 |
采用 MOLM13 和 Mv4-11。通过用 pLV218G 荧光素/慢载体(在鼠 EF1α 启动子下表达荧光素酶)转染 MOLM13 细胞,创建 MOLM13-Luc 细胞。通过使细胞通过含有浓度不断增加的索拉非尼 (1–1 nM) 的生长培养基,可以分离索拉非尼耐药的 MOLM13 (MOLM13sr) 和 Mv4-11 (Mv4-11sr)。 DNA 合成测定用于量化细胞生长。在 96 孔 Cytostar T 板中,接种 5×10 3 细胞/孔,总体积为 160 μL。将测试化合物(例如 AMG 925;0.03 和 0.3μM)连续稀释到每个孔中(20 μL/孔)后,以 20 μL/0.1 μCi 的增量添加到板的每个孔中。再孵育 72 小时后,使用 β 板计数器测量同位素掺入情况。 Vybrant 细胞凋亡检测试剂盒用于检测细胞凋亡。总之,6 孔板每孔接种 5×10 5 细胞,并应用化合物(例如 AMG 925;0.003、0.01、0.03、0.1、0.3 和 1 μM)一整天。使用试剂盒附带的试剂对细胞进行染色后,使用流式细胞术检查细胞。活细胞、凋亡细胞和死细胞的分辨率(使用 Flowjo 软件进行量化)显示在 Sytox Green 荧光与别藻蓝蛋白荧光点图中。将细胞接受 AMG 925 处理一整天后,使用 CycleTest 试剂盒分析细胞周期。将细胞用AMG 925处理24小时,然后使用CycleTest Kit分析细胞周期。使用 ModFit 软件收集 10,000 个事件,并计算每个周期阶段的细胞百分比[1]。
1. 细胞增殖实验(CCK-8法):将白血病细胞系(MOLM-13、MV4-11、THP-1)以3×10³个细胞/孔的密度接种于96孔板,过夜孵育。加入浓度为0.1 nM~1000 nM的AMG 925 (FLX925; AMG925),培养72小时后,每孔加入10 μL CCK-8试剂,继续孵育2小时。用酶标仪在450 nm处测定吸光度,以抑制增殖50%的药物浓度作为IC50[1] 2. Western blot实验:用AMG 925 (FLX925; AMG925)(1 nM~50 nM)处理MOLM-13或THP-1细胞2小时至24小时,收集细胞并用冷PBS洗涤,加入含蛋白酶和磷酸酶抑制剂的RIPA裂解液裂解细胞。采用BCA法测定蛋白浓度,取30 μg蛋白进行10% SDS-PAGE电泳,转移至PVDF膜后,用抗p-FLT3、FLT3、p-CDK4、CDK4、p-STAT5、p-ERK1/2、切割型caspase-3或GAPDH的一抗孵育;加入二抗后,用ECL试剂检测信号[1] 3. 凋亡实验(Annexin V/PI染色法):用AMG 925 (FLX925; AMG925)(1 nM~20 nM)处理THP-1细胞24小时或48小时,收集细胞并用冷PBS洗涤,重悬于结合缓冲液中,加入Annexin V-FITC和PI避光孵育15分钟,通过流式细胞仪分析凋亡细胞[1] |
| 动物实验 |
Mice: NCR-Foxn1 nu (CrTac:NCR) nude mice are employed. On the flank of NCR nude mice, 2×10 6 cells are inoculated, and they are left to grow for 13 days. AMG 925 is then given orally to mice twice a day, six hours apart, at doses of 12.5, 25, 37.5, and 50 mg/kg for ten days in a row[1].
1. Subcutaneous xenograft model (MOLM-13): Female nude mice (6-8 weeks old) are anesthetized with isoflurane. MOLM-13 cells (5×10⁶ cells in 0.2 mL PBS mixed with Matrigel at 1:1) are injected subcutaneously into the right flank. When tumors reach ~120 mm³, mice are randomly divided into 3 groups: vehicle control, AMG 925 (FLX925; AMG925) 10 mg/kg, and 25 mg/kg. The drug is formulated in 0.5% methylcellulose + 0.2% Tween 80 and administered orally once daily for 21 days. Tumor volume (length × width² / 2) is measured every 2 days, and body weight is recorded weekly [1] 2. Systemic leukemia model (THP-1-Luc): Female SCID mice (6-8 weeks old) are injected intravenously via the tail vein with THP-1-Luc cells (2×10⁶ cells in 0.2 mL PBS). Seven days after cell injection, mice are divided into 2 groups (n=8/group): vehicle control and AMG 925 (FLX925; AMG925) 25 mg/kg. The drug is administered orally once daily. Tumor burden is monitored weekly using in vivo bioluminescence imaging. Mice are euthanized when they show signs of morbidity (weight loss > 20%, lethargy) [1] |
| 药代性质 (ADME/PK) |
1. Oral pharmacokinetics in mice: Male C57BL/6 mice (n=3 per time point) are given AMG 925 (FLX925; AMG925) via oral gavage at 25 mg/kg. Blood samples are collected at 0.25, 0.5, 1, 2, 4, 8, 12, and 24 hours post-dosing. Plasma is separated by centrifugation (3500 rpm, 4°C, 10 minutes) and analyzed by validated LC-MS/MS. Key parameters: Cmax = 895 ng/mL, Tmax = 1.5 hours, AUC0-24h = 5890 ng·h/mL, t1/2 = 8.2 hours, oral bioavailability = 48% [1]
2. Tissue distribution: At 2 hours post-oral dosing (25 mg/kg), mice are euthanized, and tissues (liver, spleen, bone marrow, kidneys, lungs, brain) are collected. The highest drug concentration is found in the liver (3480 ng/g), followed by the spleen (3050 ng/g) and bone marrow (2780 ng/g). The brain concentration is 52 ng/g, indicating low blood-brain barrier penetration [1] 3. Plasma protein binding: Using the ultrafiltration method, AMG 925 (FLX925; AMG925) is spiked into mouse, rat, dog, and human plasma at 10 ng/mL and 1000 ng/mL. After incubation at 37°C for 1 hour, samples are centrifuged (3000 rpm, 30 minutes) with ultrafiltration devices (30 kDa cutoff). The protein binding rate is > 99% across all species and concentrations [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity in mice: Male and female C57BL/6 mice (n=3/sex/dose) are given AMG 925 (FLX925; AMG925) via oral gavage at 40 mg/kg, 80 mg/kg, and 160 mg/kg. No mortality is observed at 40 mg/kg or 80 mg/kg. At 160 mg/kg, 1 out of 6 mice dies within 48 hours, and surviving mice show transient weight loss (max 13% at day 3) which recovers by day 8 [1]
2. Subacute toxicity (28-day study): Mice are treated with AMG 925 (FLX925; AMG925) (10 mg/kg, 25 mg/kg, oral, once daily) for 28 days. The 10 mg/kg group shows no significant changes in body weight, clinical chemistry (ALT, AST, creatinine), or hematology (white blood cells, platelets). The 25 mg/kg group shows a slight increase in ALT (1.5-fold vs control) but no histopathological changes in the liver [1] |
| 参考文献 | |
| 其他信息 |
AMG-925 is an organic heterotricyclic compound that is 9H-pyrido[4',3':4,5]pyrrolo[2,3-d]pyrimidine which is substituted by a [6-(hydroxyacetyl)-5,6,7,8-tetrahydro-1,6-naphthyridin-2-yl]nitrilo group at position 2 and by a trans-4-methylcyclohexyl group at position 9. It is a FLT3 and CDK4 dual kinase inhibitor that has antineoplastic activity. Currently under clinical investigation in patients with relapsed or refractory acute myeloid leukemia (AML). It has a role as an antineoplastic agent, an apoptosis inducer, an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor and an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor. It is a secondary amino compound, a tertiary amino compound, a naphthyridine derivative, a primary alpha-hydroxy ketone and an organic heterotricyclic compound.
1. Therapeutic background: AMG 925 (FLX925; AMG925) is a dual-targeted kinase inhibitor developed for the treatment of hematological malignancies, especially acute myeloid leukemia (AML) driven by FLT3 mutations (e.g., FLT3-ITD) and/or CDK4 overexpression, which are associated with poor prognosis and treatment resistance [1] 2. Mechanism of action: The drug exerts anti-leukemia effects by competitively binding to the ATP-binding pockets of FLT3 and CDK4, inhibiting their autophosphorylation and downstream signaling pathways (JAK-STAT, RAS-ERK, PI3K-AKT). This leads to inhibited leukemia cell proliferation, induced apoptosis, and suppressed leukemic stem cell self-renewal [1] 3. Preclinical advantage: Compared to single-target inhibitors, AMG 925 (FLX925; AMG925) targets multiple kinases frequently mutated in AML, making it potentially effective for patients with co-mutations or resistance to single-target agents [1] |
| 分子式 |
C26H29N7O2
|
|---|---|
| 分子量 |
471.55
|
| 精确质量 |
471.238
|
| 元素分析 |
C, 66.22; H, 6.20; N, 20.79; O, 6.79
|
| CAS号 |
1401033-86-0
|
| 相关CAS号 |
AMG 925 HCl;1401034-19-2
|
| PubChem CID |
60202647
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
712.3±70.0 °C at 760 mmHg
|
| 闪点 |
384.6±35.7 °C
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
| 折射率 |
1.766
|
| LogP |
1.67
|
| tPSA |
109.06
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
747
|
| 定义原子立体中心数目 |
0
|
| SMILES |
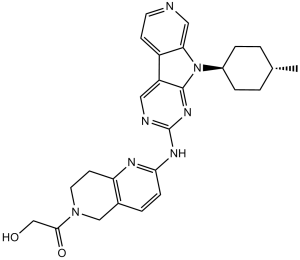
C1(NC2C=CC3CN(C(CO)=O)CCC=3N=2)N=CC2C3C=CN=CC=3N([C@H]3CC[C@H](C)CC3)C=2N=1
|
| InChi Key |
BBUVDDPUURMFOX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C26H29N7O2/c1-16-2-5-18(6-3-16)33-22-13-27-10-8-19(22)20-12-28-26(31-25(20)33)30-23-7-4-17-14-32(24(35)15-34)11-9-21(17)29-23/h4,7-8,10,12-13,16,18,34H,2-3,5-6,9,11,14-15H2,1H3,(H,28,29,30,31)
|
| 化学名 |
2-hydroxy-1-[2-[[8-(4-methylcyclohexyl)-4,6,8,11-tetrazatricyclo[7.4.0.02,7]trideca-1(9),2,4,6,10,12-hexaen-5-yl]amino]-7,8-dihydro-5H-1,6-naphthyridin-6-yl]ethanone
|
| 别名 |
FLX 925; AMG925; FLX925; FLX-925; AMG-925; AMG 925
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1207 mL | 10.6033 mL | 21.2067 mL | |
| 5 mM | 0.4241 mL | 2.1207 mL | 4.2413 mL | |
| 10 mM | 0.2121 mL | 1.0603 mL | 2.1207 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Activity of AMG 925 in subcutaneous MOLM13 xenograft tumor model.Mol Cancer Ther. 2014 Apr;13(4):880-9. |
Activity of AMG 925 in systemic MOLM13-Luc xenograft tumor model. Mol Cancer Ther. 2014 Apr;13(4):880-9. |
Activity of AMG 925 in Colo205 xenograft tumor model. Mol Cancer Ther. 2014 Apr;13(4):880-9. |