| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and almost completely absorbed from the GI tract. Peak plasma concentrations occur within 1-2 hours of oral administration of a single dose. 60-69% of a single orally administered dose of amoxapine is excreted in urine, principally as conjugated metabolites. 7-18% of the dose is excrete feces mainly as unconjugated metabolites. Less than 5% of the dose is excreted as unchanged drug in urine. Widely distributed in body tissues with highest concentrations found in lungs, spleen, kidneys, heart, and brain. Lower concentrations can be detected in testes and muscle. Metabolism / Metabolites Amoxapine is almost completely metabolized in the liver to its major metabolite, 8-hydroxyamoxapine, and a minor metabolite, 7-hydroxyamoxapine. Both metabolites are phamacologically inactive and have half-lives of approximately 30 and 6.5 hours, respectively. Amoxapine is almost completely metabolized in the liver to its major metabolite, 8-hydroxyamoxapine, and a minor metabolite, 7-hydroxyamoxapine. Both metabolites are phamacologically inactive and have half-lives of approximately 30 and 6.5 hours, respectively. Route of Elimination: 60-69% of a single orally administered dose of amoxapine is excreted in urine, principally as conjugated metabolites. 7-18% of the dose is excrete feces mainly as unconjugated metabolites. Less than 5% of the dose is excreted as unchanged drug in urine. Half Life: 8 hours Biological Half-Life 8 hours |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Liver test abnormalities occur in a small proportion of patients on long term therapy with amoxapine, but elevations are usually mild, asymptomatic and transient, reversing even with continuation of medication. Instances of clinically apparent acute liver injury without jaundice have been reported due to amoxapine, but have been quite rare. Published cases have been mild, anicteric and asymptomatic. The onset of injury was within 1 to 4 weeks of starting, and the pattern of serum enzyme elevations was hepatocellular. Immunoallergic features and autoantibody formation were not present. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with amoxapine during breastfeeding, other agents may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Two cases of galactorrhea have been reported in nonbreastfeeding women who were taking amoxapine. The clinical relevance of these findings in nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking amoxapine. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. Protein Binding In vitro tests show that amoxapine binding to human plasma proteins is approximately 90%. |
| 其他信息 |
Amoxapine can cause developmental toxicity according to state or federal government labeling requirements.
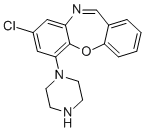
Amoxapine is a dibenzooxazepine compound having a chloro substituent at the 2-position and a piperazin-1-yl group at the 11-position. It has a role as an antidepressant, an adrenergic uptake inhibitor, a dopaminergic antagonist, a serotonin uptake inhibitor and a geroprotector. Amoxapine, the N-demethylated derivative of the antipsychotic agent loxapine, is a dibenzoxazepine-derivative tricyclic antidepressant (TCA). TCAs are structurally similar to phenothiazines. They contain a tricyclic ring system with an alkyl amine substituent on the central ring. In non-depressed individuals, amoxapine does not affect mood or arousal, but may cause sedation. In depressed individuals, amoxapine exerts a positive effect on mood. TCAs are potent inhibitors of serotonin and norepinephrine reuptake. In addition, TCAs down-regulate cerebral cortical β-adrenergic receptors and sensitize post-synaptic serotonergic receptors with chronic use. The antidepressant effects of TCAs are thought to be due to an overall increase in serotonergic neurotransmission. TCAs also block histamine H1 receptors, α1-adrenergic receptors and muscarinic receptors, which accounts for their sedative, hypotensive and anticholinergic effects (e.g. blurred vision, dry mouth, constipation, urinary retention), respectively. See toxicity section below for a complete listing of side effects. Amoxapine may be used to treat neurotic and reactive depressive disorders, endogenous and psychotic depression, and mixed symptoms of depression and anxiety or agitation. Amoxapine is a Tricyclic Antidepressant. Amoxapine is a tetracyclic antidepressant used for relief of symptoms of depression caused by either reactive or psychotic depression. Amoxapine has been associated with a low rate of minor serum aminotransferase elevations during treatment and to very rare instances of clinically apparent acute liver injury. Amoxapine is a tricyclic antidepressant of the dibenzoxazepine class. Amoxapine exerts its antidepressant effect by inhibiting the re-uptake of norepinephrine and, to a lesser degree, of serotonin, at adrenergic nerve endings and blocks the response of dopamine receptors to dopamine. This drug is used to treat symptoms of depression and may cause tardive dyskinesia. Amoxapine also binds to alpha-adrenergic, histaminergic, and cholinergic receptors which accounts for many of the side effects seen with this agent. Amoxapine, the N-demethylated derivative of the antipsychotic agent loxapine, is a dibenzoxazepine-derivative tricyclic antidepressant (TCA). TCAs are structurally similar to phenothiazines. They contain a tricyclic ring system with an alkyl amine substituent on the central ring. In non-depressed individuals, amoxapine does not affect mood or arousal, but may cause sedation. In depressed individuals, amoxapine exerts a positive effect on mood. TCAs are potent inhibitors of serotonin and norepinephrine reuptake. In addition, TCAs down-regulate cerebral cortical β-adrenergic receptors and sensitize post-synaptic serotonergic receptors with chronic use. The antidepressant effects of TCAs are thought to be due to an overall increase in serotonergic neurotransmission. TCAs also block histamine H1 receptors, α1-adrenergic receptors and muscarinic receptors, which accounts for their sedative, hypotensive and anticholinergic effects (e.g. blurred vision, dry mouth, constipation, urinary retention), respectively. See toxicity section below for a complete listing of side effects. Amoxapine may be used to treat neurotic and reactive depressive disorders, endogenous and psychotic depression, and mixed symptoms of depression and anxiety or agitation. The N-demethylated derivative of the antipsychotic agent LOXAPINE that works by blocking the reuptake of norepinephrine, serotonin, or both; it also blocks dopamine receptors. Amoxapine is used for the treatment of depression. Drug Indication For the relief of symptoms of depression in patients with neurotic or reactive depressive disorders as well as endogenous and psychotic depressions. May also be used to treat depression accompanied by anxiety or agitation. Mechanism of Action Amoxapine acts by decreasing the reuptake of norepinephrine and serotonin (5-HT). |
| 分子式 |
C17H16CLN3O
|
|---|---|
| 分子量 |
313.78
|
| 精确质量 |
313.098
|
| CAS号 |
14028-44-5
|
| 相关CAS号 |
Amoxapine-d8;1189671-27-9
|
| PubChem CID |
2170
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.37g/cm3
|
| 沸点 |
469.9ºC at 760mmHg
|
| 熔点 |
175-1760C
|
| 闪点 |
238ºC
|
| 蒸汽压 |
5.32E-09mmHg at 25°C
|
| 折射率 |
1.685
|
| LogP |
3.131
|
| tPSA |
36.86
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
424
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C(=NC1=C([H])C([H])=C([H])C([H])=C1O2)N1C([H])([H])C([H])([H])N([H])C([H])([H])C1([H])[H]
|
| InChi Key |
QWGDMFLQWFTERH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H16ClN3O/c18-12-5-6-15-13(11-12)17(21-9-7-19-8-10-21)20-14-3-1-2-4-16(14)22-15/h1-6,11,19H,7-10H2
|
| 化学名 |
8-chloro-6-piperazin-1-ylbenzo[b][1,4]benzoxazepine
|
| 别名 |
Asendin AmoxanAsendis AdisenDefanyl Demolox OxcapOxamine Amolife
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~8.33 mg/mL (~26.55 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.56 mg/mL (4.97 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 15.6 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.56 mg/mL (4.97 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 15.6 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.56 mg/mL (4.97 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1869 mL | 15.9347 mL | 31.8695 mL | |
| 5 mM | 0.6374 mL | 3.1869 mL | 6.3739 mL | |
| 10 mM | 0.3187 mL | 1.5935 mL | 3.1869 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。