| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Vasopressin/oxytocin receptor
|
|---|---|
| 体外研究 (In Vitro) |
阿托西班通过催产素阻止子宫肌层释放 IP3。子宫肌层细胞骶浆网释放的细胞内钙和通过电压门控通道的外部 Ca2+ 流入均减少。此外,当催产素存在时,阿托西班可以阻止蜕膜释放 PGE 和 PGF [1]。
催产素(OT)通过催产素受体(OTR)偶联刺激子宫收缩和促进羊膜中前列腺素/炎性细胞因子的合成,在人类分娩的开始中起着重要作用。OTR拮抗剂阿托西班被广泛用作治疗急性早产的安胎药。我们发现,在原代人羊膜细胞中,Atosiban(10μM)通过PTX敏感的Gαi发出信号,激活转录因子NF-κB p65、ERK1/2和p38,随后驱动前列腺素合成酶、COX-2和磷酸化cPLA2的上调以及前列腺素(PGE2)的排泄(n=6;p<0.05,方差分析)。此外,阿托西班治疗增加了炎性细胞因子IL-6和CCL5的表达和排泄。我们还表明,OT模拟的NF-κB、ERK1/2和p38的激活以及随后前列腺素和炎性细胞因子的合成是通过Gαi-2和Gαi-3进行的,而不是Gαq,并且不受阿托西班的抑制。激活或加重炎症不是安胎药的理想效果。因此,用于足月/早产临床管理的OT/OTR系统的治疗性调节应考虑OTR的差异性G蛋白偶联的影响以及OT或选择性OTR激动剂/拮抗剂在激活促炎途径中的作用[4]。 |
| 体内研究 (In Vivo) |
阿托西班/Atosiban影响精氨酸加压素对胎儿-母体心血管和肾脏系统的生理作用。垂体后叶激素催产素和精氨酸加压素在结构上仅存在两个氨基酸的差异。一项使用阿托西班一小时的试验并未对妊娠晚期绵羊的母亲或胎儿的心血管系统造成任何变化[1]。在小鼠的臂旁核中,阿托西班抑制表达催产素受体的神经元的激活[2]。
静脉注射阿托西班(10 nmol/kg体重)后2至8分钟达到阿托西班的峰值血浆浓度,而鼻内注射阿托西班(100 nmol/kg重量)后10至45分钟达到峰值浓度。81 Goodwin等人表明,在妊娠20至36周的女性中静脉注射Atosiban/阿托西班(300μ/min)后,在6小时内没有收缩或最大输注时间为12小时的情况下,血浆阿托西邦浓度在静脉注射开始后1小时内达到稳定状态。静脉输液前4小时子宫活动的减少与输液持续时间成正比。输注完成后,血浆阿托西班水平呈双指数下降,初始和终末半衰期分别为13±3和102±18分钟。在一项关于阿托西班对早产子宫活动(妊娠20至36周)影响的II期随机安慰剂对照试验中,输注2小时的阿托西班可导致子宫收缩频率在统计学上显著下降,表明OT在维持PTL方面发挥作用。阿托西班组报告的唯一不良后果是一名患者出现恶心和呕吐。在一项关于阿托西班对PTL影响的III期随机对照试验中(20至34周),如果在阿托西班或安慰剂输注1小时后,早产仍在继续,则允许进行安胎抢救,主要终点是分娩时间或治疗失败(需要替代安胎药的分娩进展)。两组在主要终点方面没有统计学上的显著差异。次要终点是开始阿托西班或安慰剂输注后24小时、48小时和7天成功治疗的女性比例。阿托西班组的比例明显更高:24小时时为73%对58%(P<0.001),48小时时为67%对56%(P=0.008),7天时为62%对49%(P=0.003)。与安慰剂相比,阿托西班对妊娠期延长至7天的效果在妊娠期≥28周的孕妇中更为明显;在<28周时分别为65%对48%和51%对59%。这些发现强调了VOTras在SPTL的维持中起着重要作用,但也涉及其他机制。阿托西班组的围产期死亡率为2.1%,而安慰剂组在有或没有安胎抢救的情况下为1.4%。然而,随机分组没有根据胎龄进行分层,这导致阿托西班组中晚期分娩的极早产儿过多,Cochrane Review 85因使用这些数据而受到批评。在一项随机对照试验中,与安慰剂相比,阿托西班的使用导致子宫静止时间显著延长(中位数为32.6天对27.6天)。在维持治疗期间,阿托西班组的女性注射部位反应发生率较高(70%对48%)[1]。 在经ip生理盐水处理的大鼠中,发声反应显著降低(t = 5.18; df = 14, p < 0.001) 射精(t = 5.17; p < 0.001) 与基线发声反应相比。相比之下,在接受ip阿托西班治疗的女性中,与她们的基线发声反应相比,在挂载、插入或射精过程中,对STS的发声反应没有显著降低。然而,与生理盐水治疗相比,阿托西班治疗的雌性在两次插管期间的镇痛作用显著降低(t = 3.5; df = 14, p < 0.01) 射精(t = 5.1; df = 14, p < 0.01) (图1A)。另一组未接受EB或P治疗的雌性(因此不具有性接受能力,也没有出现前凸)显示,与雄性坐骑相关的发声反应没有变化(没有发生插入或射精)。阿托西班或生理盐水或icv也得到了类似的结果。在接受生理盐水的动物中,当大鼠接受导入时,对STS的发声反应显著降低(t = 5.18; df = 14 p < 0.01),或射精(t = 5.17; df = 14, p < 0.01),与他们的基线反应相比。相比之下,接受阿托西班治疗的大鼠对内向或射精的发声反应没有显著减少(图1B)。因此,由坐骑(t = −3.2; df = 14, p < 0.05) 简介(t = −3.5; df = 14, p < 0.01) 射精(t = −5.1; df = 14, p < 0.01) 与生理盐水对照组相比,阿托西班治疗的雌性大鼠的存活率显著降低(图1B)。接受icv生理盐水的动物对引入反应的发声明显减少(t = 14.7; df = 14, p < 0.01) 射精(t = 18.8; df = 14, p < 0.01),与他们的基线反应相比。然而,在接受阿托西班静脉注射的女性中,对插入和射精的发声反应与其基线发声反应的数量没有显著差异。因此,由内向引起的镇痛作用(t = −6.9; df = 16, p < 0.01) 射精(t = −5.6; df = 16, p < 0.01) 与生理盐水对照组相比,阿托西班治疗的雌性大鼠的存活率显著降低(图1C)。Cohen的d效应大小分析显示,在所有组中,在插入或射精期间的效应大小都大于插入时的效应大小,在插入以及插入和射精期间,icv组的效应大小大于其他组(表1)[3]。 |
| 细胞实验 |
实时聚合酶链式反应(RT-PCR)[4]
根据制造商的规范,使用RNA STAT-60试剂通过硫氰酸胍-苯酚-氯仿提取法提取总RNA。在cDNA合成之前,通过DNaseI处理消除了任何DNA污染。使用SuperScriptII第一链合成试剂盒将DNaseI处理的RNA用于第一链cDNA合成。使用SYBR Green I Master mix在ABI StepOne实时PCR系统上进行实时PCR,验证基因表达。使用Primer Express软件生成的靶DNA特异性引物进行扩增。RT-PCR使用以下基因特异性引物:L19、5′-GCGAAGGGTACAGCCAAT-3′和5′-GCAGCCGGGCGCAAA-3′;COX-2、5′-TGTGCACATTGAGT-GGCT-3′和5′-ACTTTGTACTGCGGGT-G-3′;IL-6、5′-ACCTCC-AAAGATGGCTGAA-3′和5′-AGCTCTGTTCCTCAC-3′;CCL5、5′-CCATA-TCCTCGGACACCAC-3′和5′-TGTATCC-CGAACCCATTTC-3′。使用Sequence Detector Version1.7软件对数据进行分析。使用比较Ct法评估表达水平,并将目标Ct值归一化为核糖体蛋白L19进行分析。 聚合酶链式反应(PCR)[4] 使用Phusion高保真DNA聚合酶进行聚合酶链式反应(PCR)。按照制造商的方案制备PCR反应混合物。模板DNA最初在98°C下变性30秒,然后反应进行30次98°C的热循环10秒,引物在60°C下退火30秒,在72°C下延伸30秒。随后进行72°C的最后延伸步骤10分钟。然后使用1.5-2%(w/v)琼脂糖凝胶通过电泳分析PCR产物。通过使用包含已知大小的限制性片段的超加法器V来估计DNA片段的大小。这些带子是用深色阅读器拍摄的。 蛋白质提取和蛋白质印迹[4] 在由1%Triton X-100、1%脱氧胆酸钠、0.1%SDS、150 mM NaCl、10 mM Tris(pH 7.4)和1 mM EDTA与1 mM PMSF、蛋白酶和磷酸酶抑制剂混合物组成的放射免疫沉淀试验缓冲液中,细胞在冰上裂解10分钟。将溶解的样品在4°C下以13000×g的速度离心10分钟。回收所得上清液,并使用BioRad蛋白质测定试剂盒测定蛋白质浓度。总共20μg的总蛋白在80°C下变性10分钟,然后在140 V下在10%SDS聚丙烯酰胺凝胶上进行电泳分离80分钟。然后使用湿转移室系统在300 mA下将溶解的蛋白质转移到PVDF膜上90分钟。膜在4°C下在一抗(详见下文)中孵育过夜,然后在第二天与HRP偶联的二抗一起孵育。使用ECL plus进行信号检测。为了确认每个孔的负载相等,用0.2 M NaOH剥离膜10分钟,并重新探测β-肌动蛋白。 siRNA基因沉默[4] 根据制造商的方案,使用Amaxa Nucleofector技术进行基因沉默研究的转染。使用程序T-020通过电穿孔用30pmol的siGENOME SMARTpool siRNA转染细胞/siRNA悬浮液。从转染细胞中提取总蛋白,在72小时后进行进一步分析。 ELISA[4] 通过标准ELISA测定释放的IL-6、CCL5和PGE2的浓度。从处理过的羊膜培养物中收集上清液,并立即在-20°C下冷冻,以便根据制造商的说明通过ELISA进行后续分析。 |
| 动物实验 |
Drug administration [1]
In Experiment 1, Atosiban (0.5 mg/ml in saline) was administered ip at a dose of 500 μg/kg body weight. Atosiban (1 mg/kg bw) was reported to inhibit oxytocin-induced analgesia (Abbasnezhad et al., 2016). We observed that when we administered this dose of Atosiban to female rats pretreated with EB and P, their sexual behavior was inhibited. For this reason, we reduced the atosiban dose that we used to 500 μg/kg bw. At this dose, we observed that female sexual behavior was expressed normally, but the copulation-induced analgesia was significantly reduced (unpublished findings). In Experiment 2, Atosiban was administered intrathecally (it, 500 ng in 5 μl saline) according to the method of Hylden and Wilcox (Hylden and Wilcox, 1980). The drug was administrated over a period of 120 s using a 25 μl microsyringe mounted on a microinjector. The 7.5 cm length PE10 catheter contained 7 μl of saline, plus 5 μl of atosiban or saline, plus 7 μl of saline as a flush. In Experiment 3, atosiban was administered intracerebroventricularly (icv; 500 ng in 1 μl) using a 10 μl microsyringe, over a period of approximately 50–60 s, according to the method previously described (Gómora et al., 1994). Copulation-induced antinociception and effect of Atosiban [1] Saline or Atosiban was administered immediately after establishing the baseline vocalization response. Thirty minutes later, a sexually experienced male rat was introduced into the arena. A single 20% STS was given to females at the onset of each mounting train. Behavior patterns were defined as follows: a mount (M) consisted of the male rat climbing onto the female's rump and grasping the flanks with the forelegs, followed by approx. 15 to 20 rapid thrusts to the perineal region; intromission (I) was a mount motor pattern with insertion of the penis into the vagina; ejaculation (E) was an intromission motor pattern with a duration of approx. 640 ms (Beyer et al., 1981). The percent of STS applications that elicited vocalization following M, I, or E across the entire copulatory series was calculated for each animal, and the percent of females vocalizing after a single ejaculation (n = 8) was also determined. Thus, the timing of the shocks and the total number of shocks that each female received was determined by the male's behavior, rather than by a predetermined schedule, since the experimenter delivered each shock upon initiation of the mounting train. With this stimulation schedule, a single shock train was administered 100–150 msec after initiation of each copulatory event. A subgroup of sexually unreceptive rats (ovx and treated only with saline, 1 ml/kg body weight; n = 8) was also tested, in order to determine if mounting trains without intromissions affected the vocalization response in unreceptive females. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In women receiving 300 μg/min by infusion for 6-12 h, average steady state concentrations of 442 ng/mL were reached within 1 h. Steady state concentrations increase proportionally to dosage. Small amounts of atosiban are found in the urine with 50 times the amount appearing as the large fragment metabolite (des-(Orn⁸, Gly⁹-NH2)-[Mpa¹, D-Tyr(Et)², Thr⁴]-oxytocin. The amount of drug excreted in the feces is not known. Atosiban has a mean volume of distribution of 41.8 L. Atosiban crosses the placenta and, at a dose of 300 μg/min, was found to have a 0.12 maternal/fetal concentration ratio. Atosiban has a mean clearance rate of 41.8 L/h. Metabolism / Metabolites There are two metabolites of atosiban created through the cleavage of the peptide bond between ornithine and proline which is thought to be facilitated by prior cleavage of the disulfide bridge. The larger fragment remains active as an antagonist of oxytocin receptors but is 10 times less potent than the parent molecule. At a dosage of 300 μg/min the ratio of parent molecule to the main metabolite was observed to be 1.4 at the second hour and 2.8 at the end of infusion. Biological Half-Life Atosiban does not conform to either 1-compartment or 2-compartment kinetics. It has been determined to have an initial half life (tα) of 0.21 h and a terminal half life (tβ) of 1.7 h. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Atosiban is 46-48% bound to plasma proteins in pregnant women. It is not known to partition into red blood cells. Differences in the free fraction of drug between maternal and fetal compartments are unknown. |
| 参考文献 |
[1]. Sanu O, et al. Critical appraisal and clinical utility of atosiban in the management of preterm labor. Ther Clin Risk Manag. 2010 Apr 26;6:191-9.
[2]. Philip J Ryan, et al. Oxytocin-receptor-expressing Neurons in the Parabrachial Nucleus Regulate Fluid Intake. Nat Neurosci. 2017 Dec;20(12):1722-1733. [3]. Copulation-induced antinociception in female rats is blocked by atosiban, an oxytocin receptor antagonist. Horm Behav. 2019 Jan:107:76-79. [4]. The oxytocin receptor antagonist, Atosiban, activates pro-inflammatory pathways in human amnion via G(αi) signalling. Mol Cell Endocrinol. 2016 Jan 15:420:11-23. |
| 其他信息 |
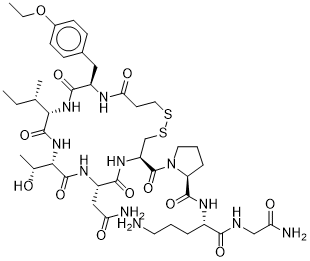
Atosiban is an oligopeptide.
Atosiban is an inhibitor of the hormones oxytocin and vasopressin. It is used intravenously to halt premature labor. Although initial studies suggested it could be used as a nasal spray and hence would not require hospital admission, it is not used in that form. Atobisan was developed by the Swedish company Ferring Pharmaceuticals. It was first reported in the literature in 1985. Atosiban is licensed in proprietary and generic forms for the delay of imminent pre-term birth in pregnant adult women. Drug Indication Atosiban is indicated for use in delaying imminent pre-term birth in pregnant adult women with: - regular uterine contractions of at least 30 s duration at a rate of at least 4 per 30 min - a cervical dilation of 1-3cm (0-3cm for nulliparas) and effacement of at least 50% - a gestational age of 24-33 weeks - a normal fetal heart rate Tractotile is indicated to delay imminent pre-term birth in pregnant adult women with: regular uterine contractions of at least 30 seconds duration at a rate of ⥠4 per 30 minutes; a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of ⥠50%; a gestational age from 24 until 33 completed weeks; a normal foetal heart rate. Atosiban is indicated to delay imminent pre-term birth in pregnant adult women with: regular uterine contractions of at least 30 seconds' duration at a rate of ⥠4 per 30 minutes; a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of ⥠50%; a gestational age from 24 until 33 completed weeks; a normal foetal heart rate. Mechanism of Action Atosiban is a synthetic peptide oxytocin antagonist. It resembles oxytocin with has modifications at the 1, 2, 4, and 8 positions. The N-terminus of the cysteine residue is deaminated to form 3-mercaptopropanic acid at position 1, at position 2 L-tyrosine is modified to D-tyrosine with an ethoxy group replacing the phenol , threonine replaces glutamine at postion 4, and ornithine replaces leucine at position 8. It binds to membrane bound oxytocin receptors on the myometrium and prevents oxytocin-stimulated increases in inositol triphosphate production. This ultimately prevents release of stored calcium from the sarcoplasmic reticulum and subsequent opening of voltage gated calcium channels. This shutdown of cytosolic calcium increase prevents contractions of the uterine muscle, reducing the frequency of contractions and inducing uterine quiescence. Atosiban has more recently been found to act as a biased ligand at oxytocin receptors. It acts as an antagonist of Gq coupling, explaining the inhibition of the inositol triphosphate pathway thought to be responsible for the effect on uterine contraction, but acts as an agonist of Gi coupling. This agonism produces a pro-inflammatory effect in the human amnion, activating pro-inflammatory signal tranducer NF-κB. It is thought that this reduces atosiban's effectiveness compared to agents which do not produce inflammation as inflammatory mediators are known to play a role in the induction of labour. Pharmacodynamics Atosiban reduces the frequency of uterine contractions to delay pre-term birth in adult females and induces uterine quiescence. Preterm birth is the major cause of perinatal morbidity and mortality in the developed world, and spontaneous preterm labor is the commonest cause of preterm birth. Interventions to treat women in spontaneous preterm labor have not reduced the incidence of preterm births but this may be due to increased risk factors, inclusion of births at the limits of viability, and an increase in the use of elective preterm birth. The role of antibiotics remains unproven. In the largest of the randomized controlled trials, evaluating the use of antibiotics for the prevention of preterm births in women in spontaneous preterm labor, antibiotics against anaerobes and bacterial vaginosis-related organisms were not included, and no objective evidence of abnormal genital tract flora was obtained. Atosiban and nifedipine are the main tocolytic agents used to treat women in spontaneous preterm labor, but atosiban is the tocolytic agent with the fewest maternal - fetal side effects. A well conducted randomized controlled trial comparing atosiban with nifedipine for their effectiveness and safety is needed.[1] Brain regions that regulate fluid satiation are not well characterized, yet are essential for understanding fluid homeostasis. We found that oxytocin-receptor-expressing neurons in the parabrachial nucleus of mice (OxtrPBN neurons) are key regulators of fluid satiation. Chemogenetic activation of OxtrPBN neurons robustly suppressed noncaloric fluid intake, but did not decrease food intake after fasting or salt intake following salt depletion; inactivation increased saline intake after dehydration and hypertonic saline injection. Under physiological conditions, OxtrPBN neurons were activated by fluid satiation and hypertonic saline injection. OxtrPBN neurons were directly innervated by oxytocin neurons in the paraventricular hypothalamus (OxtPVH neurons), which mildly attenuated fluid intake. Activation of neurons in the nucleus of the solitary tract substantially suppressed fluid intake and activated OxtrPBN neurons. Our results suggest that OxtrPBN neurons act as a key node in the fluid satiation neurocircuitry, which acts to decrease water and/or saline intake to prevent or attenuate hypervolemia and hypernatremia.[2] Aims: We hypothesized that copulation-induced temporary anti-nociception in female rats is mediated by the activation of central and/or peripheral oxytocin receptors. To test this hypothesis, we assessed the effects of intraperitoneal (ip), intrathecal (it), and intra-cerebroventricular (icv) administration of an oxytocin receptor antagonist (Atosiban), on copulation-induced temporary anti-nociception in estrous rats. Main methods: The treatment groups were ovariectomized rats pre-treated subcutaneously (sc) with 10 μg of estradiol benzoate (EB) followed 24 h later by an sc injection of 5 μg EB, and 4 h later, by an sc injection of 2 mg progesterone (P4). Rats were then administered saline vehicle (ip, it, or icv: control groups) or atosiban (500 μg/kg ip; 500 ng it; or 500 ng icv: experimental groups). Thirty minutes after drug or saline administration, their sexual behavior was tested by pairing with a sexually-experienced male rat. Brief pulse trains of 50 Hz, 300 ms duration, supra-threshold tail electrical shocks (STS) were delivered before and during copulatory activity i.e., while the female was receiving mounts, intromissions, or ejaculations, and we recorded whether vocalization occurred in response to each STS. Key findings: Replicating our previous findings, the vocalization response to STS in control rats was significantly attenuated during intromissions and ejaculations, compared to their baseline (pre-mating) response, indicative of anti-nociception. By contrast, rats pre-treated with Atosiban (each route of administration) failed to show an attenuation of the vocalization response to shock. Significance: These findings provide evidence that the temporary anti-nociceptive effect of copulation in female rats is mediated by copulation-induced release of endogenous oxytocin in brain, spinal cord and periphery.[3] Fetal concerns regarding the use of Atosiban are discussed in the literature mainly based on the results of the Atosiban versus placebo trial by Romero and co-workers (Romero et al., 2000) who found a higher rate of fetal-infant death in the Atosiban treated group in extremely premature infants. There was however a significant imbalance in randomisation at gestational ages with more very preterm infants being exposed to Atosiban. Atosiban crosses the placenta with an average fetal versus maternal ratio of 0.124 (Valenzuela et al., 1995) and concentrations of Atosiban do not appear to accumulate in the fetus. Romero and colleagues had previously hypothesized that the anti-vasopressin effects of Atosiban could have altered fetal responses to stress and therefore could have contributed to the poor outcome in the extremely preterm infants. However maternal and fetal cardiovascular parameters are not significantly altered when Atosiban is administered in pregnant sheep (Greig et al., 1993) and fetal oxygenation remains the same after Atosiban infusion in chronically instrumented baboons (Nathanielsz et al., 1997). Nevertheless, given the association between inflammation and poor neonatal outcome, activation or exacerbation of inflammation is not a desirable effect of any agent to be used in the context of acute preterm labour. It is therefore critical that therapeutics designed to modulate the OT/OTR system for the management of term and preterm labour take into account the effects of differential G-protein coupling of the OTR and the role of OT and selective OTR agonists/antagonists in the activation of pro-inflammatory pathways.[4] |
| 分子式 |
C43H67N11O12S2
|
|---|---|
| 分子量 |
994.19
|
| 精确质量 |
993.441
|
| 元素分析 |
C, 51.95; H, 6.79; N, 15.50; O, 19.31; S, 6.45
|
| CAS号 |
90779-69-4
|
| 相关CAS号 |
Atosiban acetate;914453-95-5; 90779-69-4
|
| PubChem CID |
5311010
|
| 序列 |
deamino-Cys(1)-D-Tyr(Et)-Ile-Thr-Asn-Cys(1)-Pro-Orn-Gly-NH2; deamino-cysteinyl-O4-ethyl-D-tyrosyl-L-isoleucyl-L-threonyl-L-asparagyl-L-cysteinyl-L-prolyl-L-ornithyl-glycinamide (1->6)-disulfide
|
| 短序列 |
CXITNCPXG
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
1469.0±65.0 °C at 760 mmHg
|
| 闪点 |
842.2±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.549
|
| LogP |
-3.41
|
| tPSA |
416.27
|
| 氢键供体(HBD)数目 |
11
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
68
|
| 分子复杂度/Complexity |
1770
|
| 定义原子立体中心数目 |
9
|
| SMILES |
CC[C@H](C)[C@H]1C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](CSSCCC(=O)N[C@@H](C(=O)N1)CC2=CC=C(C=C2)OCC)C(=O)N3CCC[C@H]3C(=O)N[C@@H](CCCN)C(=O)NCC(=O)N)CC(=O)N)[C@@H](C)O
|
| InChi Key |
VWXRQYYUEIYXCZ-OBIMUBPZSA-N
|
| InChi Code |
InChI=1S/C43H67N11O12S2/c1-5-23(3)35-41(63)53-36(24(4)55)42(64)50-29(20-32(45)56)38(60)51-30(43(65)54-17-8-10-31(54)40(62)49-27(9-7-16-44)37(59)47-21-33(46)57)22-68-67-18-15-34(58)48-28(39(61)52-35)19-25-11-13-26(14-12-25)66-6-2/h11-14,23-24,27-31,35-36,55H,5-10,15-22,44H2,1-4H3,(H2,45,56)(H2,46,57)(H,47,59)(H,48,58)(H,49,62)(H,50,64)(H,51,60)(H,52,61)(H,53,63)/t23-,24+,27-,28+,29-,30-,31-,35-,36-/m0/s1
|
| 化学名 |
(2S)-N-[(2S)-5-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxopentan-2-yl]-1-[(4R,7S,10S,13S,16R)-7-(2-amino-2-oxoethyl)-13-[(2S)-butan-2-yl]-16-[(4-ethoxyphenyl)methyl]-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide
|
| 别名 |
Atosiban; RWJ-22,164; RW22,164; Tractocile; RWJ22164; RW-22164; Tractocile; Atosiban; 90779-69-4; Tractocile; Antocin; Antocin II; tractocil; Orf-22164; Antocile; RWJ 22164
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~16.67 mg/mL (~16.77 mM)
DMSO : ≥ 16.67 mg/mL (~16.77 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.67 mg/mL (1.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.67 mg/mL (1.68 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.67 mg/mL (1.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0058 mL | 5.0292 mL | 10.0584 mL | |
| 5 mM | 0.2012 mL | 1.0058 mL | 2.0117 mL | |
| 10 mM | 0.1006 mL | 0.5029 mL | 1.0058 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05693688 | COMPLETED | Drug: Atosiban | Preterm Birth | Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) | 2017-12-01 | Phase 4 |
| NCT03570294 | COMPLETED | Drug: Atosiban | Premature Birth | Polish Mother Memorial Hospital Research Institute | 2014-02-01 | Not Applicable |
| NCT01493440 | COMPLETED | Drug: atosiban | Repeated Implantation Failure | An Sinh Hospital | 2011-03 | Not Applicable |
| NCT05382143 | UNKNOWN STATUS | Drug: Atosiban | Endometriosis | Radboud University Medical Center | 2022-02-01 | Phase 2 |
| NCT03904745 | UNKNOWN STATUS | Drug: Atosiban | Infertility, Female | Bezmialem Vakif University | 2020-12-21 | Not Applicable |
|
|