| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Antifungal
|
|---|---|
| 体外研究 (In Vitro) |
嘧菌酯(AZ)是一种全球农业广泛使用的广谱合成杀菌剂,但其环境归趋和效应引发关注。已知环境微生物会将其转化为主要代谢物嘧菌酯酸。本研究报道了商业化堆肥除臭剂地衣芽孢杆菌TAB7通过新型降解途径转化嘧菌酯的能力。通过电喷雾电离质谱(MS)、高分辨质谱和核磁共振波谱鉴定发现,TAB7将(E)-AZ代谢为(E)-3-氨基-2-(2-((6-(2-氰基苯氧基)嘧啶-4-基)氧基)苯基)丙烯酸甲酯(简称(E)-嘧菌酯胺)及其(Z)型异构体。稻瘟病菌生物测定显示,40 μg/mL (E)-AZ对线粒体复合体I与III间电子传递活性的抑制率为59.5±3.5%,而同等浓度(E)-嘧菌酯胺仅抑制36.7±15.1%,需80 μg/mL才能达到56.8±7.4%抑制率,表明其毒性低于母体化合物。这是首次发现细菌降解产生低毒代谢物嘧菌酯胺,并报道TAB7可促使(E)-AZ发生酶促异构化为(Z)-AZ。尽管仍需探究TAB7强化土壤微宇宙中AZ的归趋,但本研究拓展了对AZ生物转化产物的认知。[1]
通过田间试验(无可见病害)和可控环境实验,研究了杀菌剂嘧菌酯(甲氧基丙烯酸酯类)和环氧菌唑(甾醇生物合成抑制剂)对冬小麦叶际真菌、衰老和产量的影响。两个田间试验中,两种杀菌剂处理均比对照延长绿叶保留时间并增产。嘧菌酯的保绿效果优于环氧菌唑,且在一个试验中增产更显著。两种杀菌剂均减少了叶面腐生真菌的菌丝生长。叶片中针对Mycosphaerella spp.(主要为小麦叶枯病菌)的乳突形成和过敏反应频繁发生,这些防御反应可能加速衰老。嘧菌酯处理叶片的防御反应少于环氧菌唑处理和对照。温室实验中,接种链格孢和大孢枝孢加速小麦衰老,杀菌剂处理延缓了衰老但未显著增加生物量和产量。未接种条件下杀菌剂不影响衰老和产量。生长室实验中,嘧菌酯抑制腐生真菌孢子萌发和菌丝生长,环氧菌唑对孢子萌发抑制较弱但强烈抑制菌丝生长。两种杀菌剂均减少链格孢诱导的乳突形成(环氧菌唑更有效)。接种腐生菌未显著增加叶片呼吸速率(与接种非寄主大麦白粉菌相反),嘧菌酯不影响后者引发的呼吸增强,而环氧菌唑可降低该效应。推测嘧菌酯对Mycosphaerella spp.侵染的更强抑制是其田间增产效果更优的原因。[2] |
| 体内研究 (In Vivo) |
嘧菌酯是中国东北地区广泛使用的内吸性杀菌剂,但其对土壤微生物组的影响尚不明确。为此,我们研究了嘧菌酯对中国灰化土微生物群落结构与功能的影响。通过实验室批量实验,向未接触过嘧菌酯的田间土壤添加0、2、25和50 mg·kg-1的嘧菌酯。监测四种土壤酶(脲酶、蔗糖酶、磷酸酶和过氧化氢酶)以评估其对碳氮磷循环及微生物活性的影响。结果表明,35天后,低至2 mg·kg-1的嘧菌酯即可抑制脲酶、蔗糖酶和磷酸酶(水解酶类)活性,而相同浓度在多数情况下促进过氧化氢酶(氧化还原酶)活性。Biolog Ecoplate分析显示嘧菌酯抑制了不同碳源的利用。16S rRNA测序表明细菌操作分类单元丰富度和香农指数随浓度增加而降低。随着嘧菌酯浓度升高,鞘氨醇单胞菌相对丰度下降,而拟无枝酸菌和链霉菌增加。综上,灰化土施用嘧菌酯会降低水解酶活性,影响养分循环和碳利用,并改变微生物群落结构。[3]
农业集约化生产中农药持续进入土壤生态系统。本研究揭示了嘧菌酯(AZ;0.5、1和10 mg/L)经口暴露21天后对白线蚓(Enchytraeus crypticus)肠道菌群与真菌群落的剂量依赖性影响。AZ不仅诱导肠道机会致病菌增殖、降低有益菌丰度,还破坏其肠道微生态稳定性。同时观察到AZ对抗生素抗性基因(ARGs;拷贝数/细菌细胞)数量与标准化丰度的剂量效应,痕量AZ(>0且<0.085 μg/个体)可能富集肠道ARGs。通过结构方程模型推测,除可移动遗传元件和细菌群落外,肠道微生物互作可能是驱动ARGs产生与传播的另一关键因素。该研究为评估集约农业农药污染下土壤动物的肠道健康提供了新视角。[4] |
| 细胞实验 |
配制LB琼脂平板(1L超纯水中含:10g胰蛋白胨、10g NaCl、5g酵母提取物、16g琼脂),121°C灭菌20分钟。将甘油保存的TAB7菌株划线接种于LB平板,30°C培养过夜。挑取单菌落接种至含5mL LB的试管中,30°C、300次/分钟振荡培养过夜。5000×g离心5分钟收集菌体,重悬于含100ppm AZ(DMSO溶解,母液2000ppm)的新鲜LB培养基。30°C振荡培养60天,设置不加菌体的LB培养基为阴性对照。每10天取样(三重复),添加内标异丙硫环(100ppm DMSO溶液)后,用2.5mL乙酸乙酯萃取两次。离心蒸发溶剂后,残渣用甲醇复溶,经0.22μm PTFE滤膜过滤,采用Hitachi Elite LaChrom L2455 HPLC系统(配备二极管阵列检测器、自动进样器、柱温箱及PEGASIL-B ODS色谱柱4.6×250mm)分析。流动相为乙腈:水(70:30,v/v),流速1mL/min,柱温40°C,检测波长235nm。
稻瘟病菌线粒体制备 [1] 测试(E)-嘧菌酯胺和嘧菌酯对稻瘟病菌M. oryzae Ina 86-137(日本小种007.0)的杀菌活性:收集5日龄YPS液体培养基培养菌体的线粒体组分,检测NADH:细胞色素c氧化还原酶(复合体I和III)活性抑制。液氮冷冻菌体后,用裂解缓冲液(1M山梨醇、50mM柠檬酸钠,pH5.8)研磨,1000×g 4°C离心5分钟去除碎片,15000×g 4°C离心15分钟获得线粒体沉淀。沉淀重悬于测定缓冲液(0.25M蔗糖、1mM二硫苏糖醇、0.1mM EDTA、3mM Tris-HCl,pH7.4)立即用于检测。蛋白定量前用8M尿素室温处理30分钟,采用BioRad蛋白检测试剂盒测定。 复合体I和III活性测定 [1] 将40-50μg新鲜线粒体提取物悬浮于2mL测定缓冲液,加入嘧菌酯胺或嘧菌酯/AZ、0.8mM KCN、30μM水杨羟肟酸(SHAM)及100μM细胞色素c,监测548nm吸光度200秒。待吸光度稳定约50秒后,注入25μM NADH,通过吸光度上升测定细胞色素c还原活性。使用紫外-可见分光光度计25°C检测,35μg/mL抗霉素A作为阳性对照。以dAbs/min的吸光度斜率表示电子传递效率,计算化合物相对于阴性对照的抑制活性。 杀菌剂处理 [2] 两实验中设置嘧菌酯、环氧菌唑及未处理组(各12盆),每帐篷内12盆(各处理4盆)完全随机排列。采用轨道喷雾器(Teejet 8002 EVS喷嘴,200L/ha)按厂家推荐剂量施药:"Amistar"(嘧菌酯250g/ha)和"Opus"(环氧菌唑125g/ha)分别在GS 31-32、GS 37-39和GS 45-51期(>50%植株达目标生长期时)喷施三次。手持喷杆200L/ha喷雾量,在GS 31、GS 39和GS 59期进行三次施药。萨福克试验点设18个区组(6×2.5m/小区),伯克郡试验点设9个区组(12×2.5m/小区),各区内随机安排嘧菌酯、环氧菌唑或未处理组。 |
| 动物实验 |
Azoxystrobin/AZ (C22H17N3O5; CAS 131860-33-8; white powder; 95% purity) was dissolved in methanol/water (1:5) and a 1 g/L solution was made to prepare 0.5, 1, and 10 mg azoxystrobin /kg dry oat (environmentally relevant concentration) as the following treatment groups: AZ0.5, AZ1, and AZ10 (Zhang et al.,
Measurement of the soil antibiotic background value and the Azoxystrobin/AZ residue of E. crypticus [4] To characterize the background value of antibiotics in the used soil suspension, a Shimadzu liquid chromatograph coupled with ABI 3200 triple-quadruple tandem mass spectrometry was used to detect 19 types of antibiotics according to a previously published protocol (Table S3) (Hong et al., 2018). At the end of the 21-day Azoxystrobin/AZ exposure, six adult E. crypticus were used to determine the AZ residue levels using solid-phase extraction-high-performance liquid chromatography according to a previously described method (Zhang et al., 2019a, Zhang et al., 2019b). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Eight male and female rats were given 14 consecutive daily oral doses of unlabelled azoxystrobin at 1 mg/kg bw followed by a single oral dose of (14)C-pyrimidinyl-labelled azoxystrobin at 1 mg/kg bw. For the repeated doses, about 89.1% and 86.5% of the administered dose was excreted in the feces of the males and females rats within 7 days, respectively, and about 12.5% and 17.0% of the administered dose was excreted in the urine of the males and females rats within 7 days, respectively. In males and females, excretion of radioactivity was rapid, with > 96% being excreted during the first 48 hr. Approximately 0.62% and 0.39% of the administered dose was found in the carcass and tissues within 7 days after dosing in male and female rats, respectively. For the repeated dose, the highest concentrations of azoxystrobin-derived radioactivity were found in the kidneys (males and females, < 0.04 ug equivalents/g). The concentrations found in the liver were 0.02 and 0.01 ug equivalents/g for males and females, respectively. At termination, the total concentration of radioactivity in blood was 0.01 ug equivalents/g for males and females. In toxicokinetic studies, groups of male and female Alpk:APfSD rats (five to eight per group, depending on experiment) were given azoxystrobin (purity, 99%) with or without pyrimidinyl label as a single dose at 1 or 100 mg/kg bw by gavage or as 14 repeated doses of 1 mg/kg bw per day. Biliary metabolites were assessed using rats with cannulated bile ducts given a single dose at 100 mg/kg bw by gavage. The vehicle was polyethylene glycol (PEG 600) at 4 mL/kg bw. Treated rats were housed in stainless steel metabolism cages for 7 days. Urine was collected at 6 hr, and urine and feces were collected separately at 12, 24, 36, 48 h and at 24 hr intervals until 7 days after dosing. At each collection, cages were rinsed with water and cage-washing collected together with the urine. At the end of the study, cages were thoroughly rinsed with ethanol/water (1:1 v/v) and retained for radiochemical analysis. Carbon dioxide and volatiles were trapped. After 7 days, various organs and tissues were removed and analyzed for radioactivity. ... For rats receiving a single lower dose (1 mg/kg bw), total excretion of radioactivity (urine, feces, and cage wash) was 93.75% and 91.44% for males and females, respectively over the 7 days. Most (> 85%) of the urinary and fecal excretion took place during the first 36 hr after dosing. In these rats, about 83.2% and 72.6% of the administered dose was excreted in the feces of males and females within 7 days, respectively, and about 10.2% and 17.9% of the administered dose was excreted in the urine of the males and females within 7 days, respectively. Approximately 0.34% and 0.31% of the administered dose was found in the carcass and tissues within 7 days after dosing in males and females, respectively. For rats at this dose (1 mg/kg bw), the highest concentrations of radiolabel were found in the liver (mean for males and females, 0.009 ug equivalents/g) and in the kidneys (males, 0.027 ug equivalents/g; and females, 0.023 ug equivalents/g). At termination, the total concentration of radioactivity in blood was 0.004 ug equivalents/g for males and females. Less than 0.6% of the administered dose was recovered in the expired. For rats receiving the single higher dose (100 mg/kg bw), total excretion of radioactivity (urine, feces, and cage wash) was 98.29% and 97.22% for males and females, respectively, over the 7 days. Most (> 82%) of the urinary and fecal excretion took place during the first 48 hr after dosing. At this dose, about 89.37% and 84.53% of the administered dose was excreted in the feces of the males and females within 7 days, respectively, and about 8.54% and 11.54% of the administered dose was excreted in the urine of the males and females within 7 days, respectively. Approximately 0.33% and 0.33% of the administered dose was found in the carcass and tissues within 7 days after dosing in males and females rats, respectively. At this higher dose, the highest concentrations of radiolabel were found in the kidneys (males, 1.373 ug equivalents/g; and females, 1.118 ug equivalents/g) and in the liver (males, 0.812 ug equivalents/g; and females, 0.714 ug equivalents/g). At termination, the total concentration of radioactivity in blood was 0.389 ug equivalents/g for males and 0.379 ug equivalents/g for females The excretion and tissue distribution of radioactivity was investigated for 48 h in male and female rats given a single dose of azoxystrobin at 1 mg/kg bw by gavage. Treated rats were housed in metabolism cages to facilitate the collection of urine, feces, exhaled air and volatiles. One male and one female rat receiving azoxystrobin radiolabelled in each position were killed at 24 hr and 48 hr after dosing. Each carcass was frozen and sectioned in preparation for whole-body radiography. About 89% and 86% of the administered dose of (14)C-pyrimidinyl-labelled azoxystrobin was excreted within 48 hr in the urine and feces of male and female rats, respectively. Most of the radioactivity was excreted in the feces, with < 17% in the urine. The male and female rats treated with (14)C-phenylacrylate-labelled azoxystrobin excreted about 80% and 97% of the administered dose within 48 hr, respectively. Most of the radioactivity was excreted via the feces with < 21% in the urine. At 48 hr, males and females, excreted approximately 0.01% of the administered dose as carbon dioxide trap and approximately 0.01% as volatile metabolites. The male and female rats treated with (14)C-cyanophenyl- labelled azoxystrobin excreted about 95% and 98% of the administered dose within 48 hr, respectively. Most of the radioactivity was excreted via the feces, with < 16% in the urine. At 48 hr, males and females excreted small amounts of radioactivity as carbon dioxide (< 0.3%) and as volatile metabolites (0.01%). For all radiolabels, the distribution of radioactivity was similar in males and females, as shown by whole-body autoradiography. At 24 hr, most of the radiolabel was present in the alimentary canal, moderate amounts in the kidneys and small amounts in the liver. Forty-eight hours after dosing, the whole-body autoradiography results showed a marked reduction in radioactivity. The results of these studies indicated that there were no significant differences between the rates and routes of excretion or tissue distribution of azoxystrobin labelled in one of three positions. No sex-related difference in excretion profile was evident. Minor differences in excretion were primarily due to the small numbers of rats used in the study. No significant differences in the amount of radioactivity recovered in the exhaled air and as volatiles were observed between the three radiolabels or between sexes. On the basis of the results of this study, other studies of excretion and tissue retention were conducted using only pyrimidinyl-labelled azoxystrobin. Metabolism / Metabolites ... (14)C-Cyanophenyl-labelled azoxystrobin was given to bile duct cannulated and non-cannulated rats at a dose of 100 mg/kg bw. Samples of urine, feces and bile were collected for up to 72 hr. The purpose of this study was to reevaluate certain plant and goat metabolites that were previously not identified in rats and further elucidate the metabolic pathway of azoxystrobin in rats. Three further metabolites, previously detected in either plants or goats, were identified. Compound 13 (2-hydroxybenzonitrile), resulting from cleavage of the diphenyl ether link, was detected in the bile and urine as the glucoronide conjugate at a concentration of up to 1.8% of the administered dose. Compound 20 ((2-(6-(2-cyanophenoxy) pyrimidin-4-yloxy) phenyl)acetic acid) was also detected in the bile and urine at a concentration of up to 1.3%. Compound 35 (2-(2-(6-(2-cyanophenoxy) pyrimidin-4-yloxy) phenyl)glycolic acid) was detected in the urine, feces and bile at a concentration of up to 0.6%. Compounds 24 (Methyl 2-(2(6-(2-cyanophenoxy)pyrimidin-4-yloxy) phenyl)-glycolate) and 30 (2-(6-(2-cyanophenoxy) pyrimidin-4-yloxy) benzoic acid) were not detected. Bile-duct cannulated rats were given azoxystrobin radiolabelled in either the pyrimidinyl, cyanophenyl or phenylacrylate rings at 100 mg/kg bw by gavage. Comparison of the rates and routes of excretion and the profile of the metabolites showed (as previously) that there were no significant differences in the metabolism of the three differently labelled forms, thus indicating that there was minimal cleavage of the ether linkages between the aromatic rings. Experiments designed to identify metabolites were therefore conducted in bile-duct cannulated rats given (14)C-pyrimidinyl labelled azoxystrobin by gavage. In the bile-duct cannulated rats, excreta, bile, and cage wash were collected at 6, 12, 24, 36, and 48 hr and stored at -20 °C. Samples of bile, feces and urine were collected between 0 hr and 48 hr and pooled. Samples for males and females were separated. Urine and feces were collected at up to 168 hr after dosing from rats given the single dose (higher or lower) and from rats receiving repeated doses for 14 days, and were used for quantification of metabolites. Some bile samples were enzymatically digested using cholylglycine hydrolase at 30 units/mL, pH 5.6 at 37 °C overnight. Metabolites were identified using various analytical techniques, such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), proton nuclear magnetic resonance spectroscopy (NMR) and mass spectrophotometry (MS). On the basis of biliary excretion data for rats given a single dose of either (14)C-pyrimidinyl-, (14)C-phenylacrylate-, or (14)C-cyanophenyl-labelled azoxystrobin at 100 mg/kg bw, 74.4% (males) and 80.7% (females) of the pyrimidinyl-derived radioactivity was excreted in the bile after 48 hr. For the cyanophenyl-derived radioactivity, 56.6% and 62.5% was excreted in the bile of males and females, respectively. For the phenylacrylate-derived radioactivity, 64.4% (males) and 63.6% (females) was excreted in the bile. Quantitatively, there were no significant differences in biliary excretion between males and females. Azoxystrobin was found to undergo extensive metabolism in rats. A total of 15 metabolites were detected in the excreta and subsequently identified. Seven additional metabolites were detected but not identified. None of the unidentified metabolites represented more than 4.9% of the administered dose. The quantitative data for the various metabolites in the faeces, urine and bile of rats receiving a single dose of azoxystrobin at 100 mg/kg bw ... . The mass balance for the study of metabolite identification indicated that a substantial percentage of the administered radiolabel (45.6-73.6%) was unaccounted for, although the studies of excretion showed total recovery of 91.75-103.99%, with 72.6-89.3% being in the feces. The percentage of unaccounted-for radiolabel was especially notable in the groups receiving a single lower dose and a repeated lower dose. The study authors indicated that the variable efficiency in recovery could be explained by the fact that, for metabolite identification, feces were extracted with acetonitrile which allowed partitioning of the parent compound when it was present in the faeces (i.e. rats receiving the higher dose). For the groups receiving a single lower dose or repeated lower dose (where quantities of the parent compound were minimal), most of the faecal radiolabel was associated with polar metabolites that would not be present in the acetonitrile extract. The resulting concentration of radiolabel in the extract would, therefore, be very low. For the group receiving the higher dose, greater amounts of parent compound were left unabsorbed, thereby resulting in greater amounts of parent compound available for partitioning into the acetonitrile extract. The glucuronide conjugate (metabolite V) was the most prevalent biliary metabolite in both males (29.3%) and females (27.4%). Metabolite I (parent compound) was not detected in the bile. Each of the other biliary metabolites accounted for between 0.9% and 9.0% of the administered dose. In the bile-duct cannulated rats, about 15.1% and 13.6% of the faecal radioactivity was metabolite I (parent compound) in male and female rats, respectively. No parent compound was detected in the urine of bile-duct cannulated male and female rats. The predominant metabolite in the urine of the bile-duct cannulated rats was unidentified metabolite 2, which accounted for about 1.8% and 2.0% of the administered dose in male and female rats, respectively. There was no evidence for a dose-influencing metabolism, but a sex-specific difference in biotransformation was observed, with females producing more metabolites than did males. Biotransformation was unaffected by dose. The study authors suggested that absorption was dose-dependent. The oral absorption at 1 mg/kg bw was nearly complete (100%) since no parent compound was detected. The oral absorption at the higher dose (100 mg/kg bw) was estimated to be approximately 74-81% since about 19-26% of the parent compound was detected. However, it is difficult to estimate the true oral absorption value owing to poor recoveries after extraction, especially at the lower dose. ... There were two principal metabolic pathway: hydrolysis to the methoxyacid, followed by glucuronide conjugation to give metabolite V; and glutathione conjugation of the cyanophenyl ring followed by further metabolism via a number of intermediates (VI, VII, and VIII) to the mercapturic acid metabolite IX. Azoxystrobin was also hydroxylated at the 8 and 10 positions on the cyanophenyl ring followed by glucuronide conjugation (metabolites II, III, IVa and IVb). There were several minor pathways involving the acrylate moiety, resulting in formation of the metabolite XIII and XIV. Three metabolites (X, XII, and XV) arising via the cleavage of the ether linkages were identified. The metabolic fate of [(14)C]-methyl-(E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl]-3-methoxyacrylate (azoxystrobin) was determined in the male and female rat following a single oral dose of 1 and 100 mg x kg(-1) and in surgically prepared, bile duct-cannulated rats following a single oral dose of 100 mg x kg(-1). 2. Azoxystrobin was extensively metabolized with at least 15 metabolites. There was a sex difference, with females producing more metabolites than males. 3. The two principal metabolic pathways were hydrolysis of the methoxyacid followed by glucuronic acid conjugation and glutathione conjugation of the cyanophenyl ring followed by further metabolism leading to the mercapturic acid. There were also several other minor pathways. Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (L96) |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (L97) Toxicity Data LC50 (rat) > 4670 mg/m3 Non-Human Toxicity Values LD50 Rat oral >5000 mg/kg LD50 Rat percutaneous >2000 mg/kg Toxicity Data LC50 (rat) > 4670 mg/m3 Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/ /SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/ /SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/ Human Toxicity Excerpts /GENOTOXICITY/ In vitro chromosome aberrations in human lymphocytes assay: The test was positive for the induction of chromosomal aberrations in both the presence and absence of S9 activation at doses (5-50 ug/mL +S9) that were moderately to severely cytotoxic (ie, > or = 16-70% reductions in mitotic cells, respectively). |
| 参考文献 |
|
| 其他信息 |
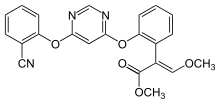
Azoxystrobin is an aryloxypyrimidine having a 4,6-diphenoxypyrimidine skeleton in which one of the phenyl rings is cyano-substituted at C-2 and the other carries a 2-methoxy-1-(methoxycarbonyl)vinyl substituent, also at C-2. An inhibitor of mitochondrial respiration by blocking electron transfer between cytochromes b and c1, it is used widely as a fungicide in agriculture. It has a role as a mitochondrial cytochrome-bc1 complex inhibitor, a xenobiotic, an environmental contaminant, an antifungal agrochemical and a quinone outside inhibitor. It is a nitrile, an aryloxypyrimidine, an enoate ester, an enol ether, a methyl ester and a methoxyacrylate strobilurin antifungal agent.
Azoxystrobin is a methoxyacrylate analog and a strobilurin fungicide. Azoxystrobin (brand name Amistar, Syngenta) is a fungicide commonly used in agriculture. Azoxystrobin possesses the broadest spectrum of activity of all known antifungals. The substance is used as an active agent protecting plants and fruit/vegetables from fungal diseases. Azoxystrobin binds very tightly to the Qo site of Complex III of the mitochondrial electron transport chain, thereby ultimately preventing the generation of ATP. Azoxystrobin is widely used in farming, particularly in wheat farming. Mechanism of Action Mode of action: fungicide with protectant, eradicant, translaminar & systemic properties. Powerfully inhibits spore germination &, in addition to its ability to inhibit mycelial growth, also shows antisporulant activity. Acts by inhibiting mitochondrial respiration by blocking electron transfer between cytochrome b & cytochrome c1. Controls pathogenic strains resistant to the 14 demethylase inhibitors, phenylamides, dicarboxamides or benzimidazoles. In this work, we showed that B. licheniformis strain TAB7 can transform (E)-AZ into methyl (E)-3-amino-2-(2-((6-(2-cyanophenoxy)pyrimidin-4-yl)oxy)phenyl)acrylate ((E)-azoxystrobin amine in short) and its (Z)-isomer. Additionally, novel AZ degradation/transformation products are reported for the first time. (Z)-AZ is possibly an enzymatic reaction product of AZ degradation, highlighting the involvement of isomerases in the transformation process. The results from this study add to the list of known AZ degradation metabolites. Although it is still unknown whether TAB7 can produce the same metabolites in environments where AZ is present in lower concentrations, the discovery of these novel degradation products indicates that further studies on the potential effects and fates of these products in the environment are necessary and will be addressed in future studies. [1] The field trials conducted in this study showed that extensive fungal–plant interactions detectable only by microscopy do occur on fungicide-treated wheat leaves, even though only < 1% leaf area was visibly diseased. The high frequency of defence reactions against attempted fungal infection makes it highly probable that the associated energy expenditure can adversely influence the final yield. The reduction of defence reactions obtained with azoxystrobin treatments, particularly compared with treatment with epoxiconazole, can therefore be part of the explanation for the superior green leaf conservation and yield recorded for azoxystrobin-treated plots compared with epoxiconazole-treated plots. The present results demonstrate an example of fungal control, observable only by microscopy, with a probable influence on yield that otherwise might have been attributed to other factors. [2] In this study, we investigated the effect of azoxystrobin on the microbial diversity and the utilization of carbon sources in Spodosols. The activities of three hydrolytic enzymes (urease, invertase and phosphatase) decreased and catalase activity increased. The AWCD values and the utilization of six classes of carbon sources decreased. In general, as azoxystrobin concentrations increased, the enzyme activity decreased. These results suggest that microbes essential to cycling soil C, N, and P were inhibited by azoxystrobin and its metabolites. The bacterial communities of soil treated with higher azoxystrobin concentration (AZO 2 and AZO 3) changed much more than control and AZO 1. Thus, the concentration of fungicide applied to soils is important to consider when considering soil microbiome impacts. Although azoxystrobin is considered to have low toxicity, these findings suggest that persistent azoxystrobin application can impact bacterial soil communities, which may inhibit important soil nutrient cycling to sustain productive croplands. However, it must be noted that this study focused on Spodosols, thus impacts to other soils may vary depending on microbial communities present and various other soil parameters. Future work should focus on quantifying soil nutrient impacts and determining safe doses of azoxystrobin to ensure long term soil health and productivity. [3] This study showed that oral exposure of AZ severely interferes with the structure and composition of bacteria and fungi in the gut of E. crypticus, inducing the growth of opportunistic pathogens and reducing the relative abundance of beneficial bacteria as well as destroying the stability of the gut microecology of E. crypticus. It is revealed that the environmental dose of AZ threatened the gut healthy of E. crypticus. Meanwhile, AZ presented a dose-dependent effect on the normalized abundance and number of ARGs by changing the gut microbiota of E. crypticus. The result also concluded that the trace dose of AZ (> 0 and < 0.085 μg/individual) might enrich the ARGs in the gut of E. crypticus. Moreover, SEM showed that the B/F richness significantly correlated with the ARG abundance (copies/bacterial cell), suggesting that the interaction of bacteria and fungi in the gut of E. crypticus may be a key contributor to the shifts in the number and abundance of ARGs (copies/bacterial cell). These findings provided new perspectives for assessing the gut health of soil fauna and a theoretical basis for guiding the use of pesticides in intensive agricultural production.[4] |
| 分子式 |
C22H17N3O5
|
|---|---|
| 分子量 |
403.39
|
| 精确质量 |
403.116
|
| 元素分析 |
C, 65.50; H, 4.25; N, 10.42; O, 19.83
|
| CAS号 |
131860-33-8
|
| 相关CAS号 |
143130-94-3; 131860-33-8
|
| PubChem CID |
3034285
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
581.3±50.0 °C at 760 mmHg
|
| 熔点 |
118 - 119ºC
|
| 闪点 |
305.3±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.626
|
| LogP |
5.13
|
| tPSA |
103.56
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
646
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C1C([H])=C(N=C([H])N=1)OC1=C([H])C([H])=C([H])C([H])=C1C#N)C1=C([H])C([H])=C([H])C([H])=C1/C(=C(/[H])\OC([H])([H])[H])/C(=O)OC([H])([H])[H]
|
| InChi Key |
WFDXOXNFNRHQEC-GHRIWEEISA-N
|
| InChi Code |
InChI=1S/C22H17N3O5/c1-27-13-17(22(26)28-2)16-8-4-6-10-19(16)30-21-11-20(24-14-25-21)29-18-9-5-3-7-15(18)12-23/h3-11,13-14H,1-2H3/b17-13+
|
| 化学名 |
methyl (E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yl]oxyphenyl]-3-methoxyprop-2-enoate
|
| 别名 |
Azoxystrobine; Heritage; Amistar; Azoxystrobin; 131860-33-8; Icia-5504; CHEBI:40909; Quadris; Bankit; Brand name: Syngenta
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.20 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.20 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4790 mL | 12.3950 mL | 24.7899 mL | |
| 5 mM | 0.4958 mL | 2.4790 mL | 4.9580 mL | |
| 10 mM | 0.2479 mL | 1.2395 mL | 2.4790 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。