| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Mps1/monopolar spindle 1 (IC50 = 0.63 nM)
|
|---|---|
| 体外研究 (In Vitro) |
BAY1217389 (BAY-1217389) 抑制 Mps1 激酶活性,IC50 值低于 10 nM,同时表现出优异的选择性。在细胞机制测定中,BAY 1217389 消除诺考达唑诱导的 SAC 活性并诱导有丝分裂过早退出,导致多核和肿瘤细胞死亡。 BAY 1217389 在体外有效抑制肿瘤细胞增殖[1]。
BAY 1161909和BAY1217389抑制Mps1激酶活性,IC50值低于10 nmol/L,同时显示出优异的选择性。在细胞机制分析中,两种Mps1抑制剂都消除了诺考达唑诱导的SAC活性,并诱导了有丝分裂的过早退出(“有丝分裂突破”),导致多核性和肿瘤细胞死亡。这两种化合物在体外均能有效抑制肿瘤细胞增殖(IC50 nmol/L范围)。[1] |
| 体内研究 (In Vivo) |
BAY1217389 (BAY-1217389) 在肿瘤异种移植研究中的单一疗法中取得了中等疗效。然而,根据其独特的作用模式,当与紫杉醇联合使用时,低剂量的 Mps1 抑制剂可减少紫杉醇诱导的有丝分裂停滞,同时减弱 SAC 活性。因此,在广泛的异种移植模型(包括那些表现出获得性或内在紫杉醇耐药性的模型)中,联合治疗在各自的 MTD 上比紫杉醇或 Mps1 抑制剂单一治疗大大提高了疗效。 BAY 1217389 显示出良好的耐受性,且不会增加紫杉醇单一疗法的毒性[1]。
在体内,Empesertib(BAY1161909)和BAY1217389在肿瘤异种移植物研究中的单一疗法中取得了中等疗效。然而,根据其独特的作用方式,当与紫杉醇联合使用时,低剂量的Mps1抑制剂通过削弱SAC活性来减少紫杉醇诱导的有丝分裂阻滞。因此,在广泛的异种移植物模型中,包括那些显示获得性或内在紫杉醇耐药性的模型中,联合治疗在各自的MTD上比紫杉醇或Mps1抑制剂单药治疗有显著提高的疗效。两种Mps1抑制剂均表现出良好的耐受性,且不会增加紫杉醇单一疗法的毒性。这些临床前研究结果验证了癌症治疗中废除SAC的创新概念,并证明了临床概念验证研究的合理性,这些研究评估了Mps1抑制剂BAY 1161909和BAY 1217389与抗有丝分裂癌症药物联合使用,以提高其疗效并潜在地克服耐药性[1]。 |
| 酶活实验 |
通过生物素化肽 (Biotin-Ahx-PWDPDDADITEILG-NH2) 的磷酸化,基于 TRFRET 的体外激酶测定可评估 BAY 1161909 或 BAY 1217389 对重组人 Mps1 的抑制作用。标准测定条件要求在两次孵育之间有 15 分钟的预孵育期。激酶和测试化合物,然后添加底物和 10 μM ATP 以启动酶反应。
激酶测定[1] 通过生物素化肽(生物素-Ahx-PWDPDDADITITEG-NH2)的磷酸化,在基于TR-FRET的体外激酶测定中评估了Empesertib(BAY1161909)或BAY1217389对重组人Mps1的抑制作用。激酶和测试化合物预孵育15分钟,然后通过添加底物和10μmol/L的ATP开始酶反应。有关更多详细信息,请参阅补充材料和方法。 激酶选择性分析[1] 使用Millipore激酶或DiscoveRx分析器筛选,对Empesertib(BAY1161909)和BAY1217389进行了针对一组激酶的反筛选。Empesertib(BAY1161909)最初在DiscoveRx激酶组中以1μmol/L的浓度进行测试,然后测定11种激酶的KD(补充表S1)。在Millipore激酶面板中以10μmol/L的浓度测试BAY 1161909,然后以1和0.1μmol/L的剂量重新测试,并测定JNK1alpha、JNK2alpha和JNK3的IC50(补充表S1)。BAY 1217389最初在DiscoveRx激酶面板中以1、0.1和0.01μmol/L的浓度进行测试(补充表S2)。 |
| 细胞实验 |
将细胞以每孔 1,000 至 5,000 个细胞的密度接种到 96 孔板中,并在补充有 10% FCS 的适当培养基中进行。 24 小时后,用 BAY1217389 (BAY-1217389) 或 BAY 1161909 的系列稀释液一式四份处理细胞。再过 96 小时后,用戊二醛固定贴壁细胞并用结晶紫染色。 IC50 值通过 4 参数拟合计算[1]。
多核试验。[2] 将U-2 OS(骨肉瘤ATCC:HTB-96)细胞以每孔2500个细胞的密度接种在20μL细胞培养基中的384孔微量滴定板中,并在37°C下孵育过夜。以100nmol/L的终浓度分三次加入恩帕替布(BAY1161909)或BAY1217389。在试验化合物存在下,细胞在37°C下处理0、24、48和72小时。此后,用4%(v/v)PFA固定细胞,用0.5%(v/v”)Triton X-100渗透,并用0.5%(v/v”)BSA在PBS中封闭。通过抗体标记检测α-微管蛋白结构。用山羊IgG阻断后,在阻断液中加入二抗。用PBS洗涤细胞,用Hoechst 33342标记细胞核。最后,洗涤细胞,用PBS覆盖,并在4°C下储存,直至图像采集。图像是用PerkinElmer Opera高内容分析阅读器获取的。 |
| 动物实验 |
Mice: Female athymic NMRI nu/nu mice, aged 50 days, with an average body weight of 20-22 g, are utilized for tumor xenograft studies. Animals are randomized to treatment and control groups (8–10 mice / group) when tumors reach a size of 20–40 mm2, depending on the growth of the tumor model. They are then treated p.o. with vehicle (70% polyethylene glycol 400, 5% ethanol, 25% solutol), BAY 1161909, BAY1217389, and/or paclitaxel. A2780cis tumor-bearing female NMRI nude mice are treated with paclitaxel (i.v., once at a dose of 24 mg/kg), BAY 1161909 (p.o., twice daily for two days at a dose of 2.5 mg/kg), and in combination with paclitaxel (i.v., once at a dose of 24 mg/kg) and BAY 1161909 (p.o., twice daily for two days at a dose of 1 mg/kg). This allows for the analysis of polyploidy and multinuclearity induction in vivo.
Pharmacokinetic investigations [2] Pharmacokinetic studies were performed in male Wistar rats and female CD1 or NMRI nu/nu mice. For i.v. studies in rats and mice, Empesertib (BAY1161909) was solubilized in 1% DMSO, 99% plasma; for p.o. studies in rats in 50% polyethylene glycol (PEG) 400, 10% ethanol, 40% water, and for p.o. studies in mice in 75% PEG 400, 5% ethanol, and 25% solutol. BAY1217389 was solubilized in 50% PEG 400, 10% ethanol, and 40% water for intravenous and p.o. dosing in rats and mice. In pharmacokinetic studies, plasma samples were collected after 2, 5, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after intravenous application and after 8, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after p.o. administration and precipitated with ice-cold acetonitrile (1:5). Supernatants were analyzed via LC/MS-MS. Pharmacokinetic parameters were estimated from the plasma concentration data, e.g., using the log-linear trapezoidal rule for AUC estimation. Maximal plasma concentrations (Cmax) and time thereof (Tmax) were taken directly from the concentration time profiles. Animal efficacy studies [2] For tumor xenograft studies, female athymic NMRI nu/nu mice (Taconic), 50 days old, average body weight 20 to 22 g, were used after an acclimatization period of 14 days. Feeding and drinking was ad libitum 24 hours per day. Human tumor cells derived from exponentially growing cell cultures were resuspended for A2780cis, NCI-H1299, and SUM-149 models in 100% Matrigel to a final concentration of 2 × 107, 3 × 107, or 5 × 107 cells/mL, respectively. Subcutaneous implants of 0.1 mL of 2 × 106 A2780cis, 3 × 106 NCI-H1299, or 5 × 106 SUM-149 cells were inoculated into the inguinal region of athymic mice. Tumor fragments of patient tumor explants MAXF 1384 or LU384, obtained from serial passage in nude mice, were cut into fragments of 4 to 5 mm diameter and transplanted subcutaneously into the flank of athymic mice. Tumor area (product of the longest diameter and its perpendicular), measured with a caliper, and body weight were determined two to three times a week. Tumor growth inhibition is presented as treatment/control ratio (T/C) calculated with tumor areas at the end of the study. Animal body weight was used as a measure for treatment-related toxicity. Body weight loss > 20% was dedicated as toxic. When tumors reached a size of approximately 20 to 40 mm², depending on growth of the tumor model, animals were randomized to treatment and control groups (8–10 mice/group) and treated p.o. with vehicle (70% PEG 400, 5 % ethanol, and 25% Solutol), Empesertib (BAY1161909), BAY1217389, and/or paclitaxel, as indicated in Tables and Figure legends. In combination treatment groups, Mps1 inhibitor and paclitaxel were applied at the same day within a time frame of 1 hour. The treatment of each animal was based on individual body weight. In vivo mode of action studies [2] For analysis of polyploidy and multinuclearity induction in vivo, A2780cis tumor–bearing female NMRI nude mice (see above) were treated with paclitaxel (intravenously once with 24 mg/kg), Empesertib (BAY1161909) (p.o. twice daily for 2 days with 2.5 mg/kg), and in combination with paclitaxel (i.v. once 24 mg/kg) and Empesertib (BAY1161909) (p.o. twice daily for 2 days 1 mg/kg). Treatment for all groups started at a tumor size of 60 mm² at day 14 after tumor cell inoculation. Tumor samples were prepared 4 and 8 hours after first Empesertib (BAY1161909) application at treatment day 1, as well as 4, 8, and 24 hours after first application of BAY 1161909 on treatment day 2. At each time point, 3 animals per treatment group were analyzed. Tumors were used for histologic examination after paraffin embedding and hematoxylin and eosin staining. |
| 药代性质 (ADME/PK) |
In vivo pharmacokinetic parameters [1]
Pharmacokinetic parameters were determined in mouse and rat. Following intravenous administration of BAY 1161909 as bolus of 0.5 mg/kg to male CD1 mouse and 0.5 mg/kg to male Wistar rat, the compound exhibited low blood clearance. The volume of distribution (Vss) was high in both species and terminal half-lives were long. After oral administration of 1 mg/kg to female NMRI mouse and 0.5 mg/kg to male Wistar rat, peak plasma levels were reached after 4 hours. The oral bioavailability was moderate in mouse and rat (Table 1). BAY1217389 was administered intravenously as bolus of 1.0 and 0.5 mg/kg to female CD1 mouse and male Wistar rat, respectively. Blood clearance was found to be low in the tested species. Vss was high and terminal half-lives were long. BAY1217389 was administered orally to female NMRI mouse (1 mg/kg) and male Wistar rat (0.5 mg/kg). Peak plasma concentrations were observed between 1.5 and 7 hours. Oral bioavailability was high in rat and moderate in mouse (Table 1). Our data demonstrate that we have identified two novel Mps1 kinase inhibitors with a favorable pharmacokinetic profile, supporting further development for clinical application. |
| 毒性/毒理 (Toxicokinetics/TK) |
Purpose: Monopolar spindle 1 (MPS1) kinase inhibitor, BAY 1217389 (BAY) synergizes with paclitaxel. This phase I study assessed the combination of BAY with paclitaxel using a novel randomized continuous reassessment method (rCRM) to improve dose determination.[2]
Patients and methods: Patients with solid tumors were randomized to receive oral BAY (twice daily 2-days-on/5-days-off) with weekly paclitaxel (90 mg/m2) or paclitaxel monotherapy in cycle 1. Dose escalation was guided by CRM modeling. Primary objectives were to assess safety, establish the MTD of BAY, and to evaluate the pharmacokinetic profiles for both compounds. Simulations were performed to determine the contribution of the rCRM for dose determination. [2] Results: In total, 75 patients were enrolled. The main dose-limiting toxicities were hematologic toxicities (55.6%). The MTD of BAY was established at 64 mg twice daily with paclitaxel. Inclusion of a control arm enabled the definitive attribution of grade ≥3 neutropenia to higher BAY exposure [AUC0-12 (P< 0.001)]. After determining the MTD, we included 19 patients with breast cancer at this dose for dose expansion. Other common toxicities were nausea (45.3%), fatigue (41.3%), and diarrhea (40.0%). Overall confirmed responses were seen in 31.6% of evaluable patients. Simulations showed that rCRM outperforms traditional designs in determining the true MTD. [2] Conclusions: The combination of BAY with paclitaxel was associated with considerable toxicity without a therapeutic window. However, the use of the rCRM design enabled us to determine the exposure-toxicity relation for BAY. Therefore, we propose that the rCRM could improve dose determination in phase I trials that combine agents with overlapping toxicities. |
| 参考文献 |
|
| 其他信息 |
Monopolar spindle 1 (Mps1) has been shown to function as the key kinase that activates the spindle assembly checkpoint (SAC) to secure proper distribution of chromosomes to daughter cells. Here, we report the structure and functional characterization of two novel selective Mps1 inhibitors, BAY 1161909 and BAY 1217389, derived from structurally distinct chemical classes. BAY 1161909 and BAY 1217389 inhibited Mps1 kinase activity with IC50 values below 10 nmol/L while showing an excellent selectivity profile. In cellular mechanistic assays, both Mps1 inhibitors abrogated nocodazole-induced SAC activity and induced premature exit from mitosis ("mitotic breakthrough"), resulting in multinuclearity and tumor cell death. Both compounds efficiently inhibited tumor cell proliferation in vitro (IC50 nmol/L range). In vivo, BAY 1161909 and BAY 1217389 achieved moderate efficacy in monotherapy in tumor xenograft studies. However, in line with its unique mode of action, when combined with paclitaxel, low doses of Mps1 inhibitor reduced paclitaxel-induced mitotic arrest by the weakening of SAC activity. As a result, combination therapy strongly improved efficacy over paclitaxel or Mps1 inhibitor monotreatment at the respective MTDs in a broad range of xenograft models, including those showing acquired or intrinsic paclitaxel resistance. Both Mps1 inhibitors showed good tolerability without adding toxicity to paclitaxel monotherapy. These preclinical findings validate the innovative concept of SAC abrogation for cancer therapy and justify clinical proof-of-concept studies evaluating the Mps1 inhibitors BAY 1161909 and BAY 1217389 in combination with antimitotic cancer drugs to enhance their efficacy and potentially overcome resistance. Mol Cancer Ther; 15(4); 583-92.
|
| 分子式 |
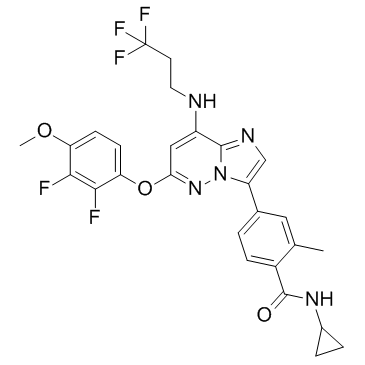
C27H24F5N5O3
|
|---|---|
| 分子量 |
561.5032
|
| 精确质量 |
561.179
|
| 元素分析 |
C, 57.75; H, 4.31; F, 16.92; N, 12.47; O, 8.55
|
| CAS号 |
1554458-53-5
|
| 相关CAS号 |
1554458-53-5
|
| PubChem CID |
78320750
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.617
|
| LogP |
4.64
|
| tPSA |
89.78
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
867
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC(C([H])([H])C([H])([H])N([H])C1=C([H])C(=NN2C1=NC([H])=C2C1C([H])=C([H])C(=C(C([H])([H])[H])C=1[H])C(N([H])C1([H])C([H])([H])C1([H])[H])=O)OC1C([H])=C([H])C(=C(C=1F)F)OC([H])([H])[H])(F)F
|
| InChi Key |
WNEILUNVMHVMPH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H24F5N5O3/c1-14-11-15(3-6-17(14)26(38)35-16-4-5-16)19-13-34-25-18(33-10-9-27(30,31)32)12-22(36-37(19)25)40-21-8-7-20(39-2)23(28)24(21)29/h3,6-8,11-13,16,33H,4-5,9-10H2,1-2H3,(H,35,38)
|
| 化学名 |
N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-(3,3,3-trifluoropropylamino)imidazo[1,2-b]pyridazin-3-yl]-2-methylbenzamide
|
| 别名 |
BAY-1217389; BAY 1217389; 1554458-53-5; BAY1217,389; BAY-1217,389; Bay 1217,389; Benzamide, N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-[(3,3,3-trifluoropropyl)amino]imidazo[1,2-b]pyridazin-3-yl]-2-methyl-; M964LB1114; N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-(3,3,3-trifluoropropylamino)imidazo[1,2-b]pyridazin-3-yl]-2-methylbenzamide; UNII-M964LB1114; BAY1217389
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~178.1 mM)
Ethanol: ~8 mg/mL (~14.3 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (4.45 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7809 mL | 8.9047 mL | 17.8094 mL | |
| 5 mM | 0.3562 mL | 1.7809 mL | 3.5619 mL | |
| 10 mM | 0.1781 mL | 0.8905 mL | 1.7809 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02366949 | Completed | Drug: BAY1217389 Drug: Paclitaxel |
Medical Oncology | Bayer | February 27, 2015 | Phase 1 |