| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
由于生物胞素与亲和素具有很强的亲和力,因此可以在光显微镜和电子显微镜水平上看到多种亲和素标记物。使用生物胞素可以看到树突和轴突树枝化的出现[1]。兔视网膜中的生物胞素宽视场双极细胞具有巨大的树突状乔木和少量的 ON 视锥细胞双极细胞,因此不会与其树突区域内的每个视锥细胞接触。使用抗蓝视锥细胞视蛋白和红绿视锥细胞视蛋白的抗体来鉴定视锥细胞类型,之后通过选择性吸收生物胞素并用花生凝集素标记视锥细胞来鉴定双极细胞。生物胞素标记的细胞会避开不被蓝色视锥细胞视蛋白染色的视锥细胞,并选择性地接触其外节可染色的视锥细胞。生物胞素广域双极细胞是先前在灵长类动物、小鼠和地松鼠视网膜中发现的蓝锥双极细胞的同源物,是在兔子视网膜中发现的 ON 蓝锥双极细胞[3]。
|
| 体内研究 (In Vivo) |
大脑的主要特征之一是其在不同空间尺度上的密集连接性。理解大脑功能显然首先需要全面描述神经元解剖连接。因此多年来开发了大量可用于追踪单突触或多突触连接的组织学标记物并不令人意外。生物胞素是一种常用于绘制脑连接图谱的经典神经解剖示踪剂。然而该分子在生物素酶作用下的内源性降解使其无法适用于长期实验,并限制了所呈现连接的质量和完整性。为了提高这一经典示踪剂的稳定性,我们设计并合成了两种新型生物胞素衍生物。本文展示了这些化合物在生物组织中显著提升的稳定性,同时保留了作为神经元示踪剂的功能。注射后24小时和96小时的实验证明,与传统生物胞素相比,新合成分子能提供更详细、更完整的脑网络信息。初步结果表明,所报道的分子设计可进一步多样化,用于磁共振成像与光学或电子显微镜相结合的多模态示踪实验。[1]
通过对东方铃蟾(Bombina orientalis)进行顺行与逆行生物胞素标记及细胞内生物胞素注射(共标记148个神经元或神经元集群),我们研究了除背内侧皮层外端脑中枢的连接性与细胞构筑。研究发现端脑可分为以下区域:(1)中腹侧端脑尾部的中央杏仁核-终纹床核,与内脏自主神经中枢相连;(2)腹侧端脑尾外侧部的犁鼻器杏仁核,接收副嗅球输入并主要投射至视前区/下丘脑;(3)位于犁鼻器杏仁核外侧端脑尾极的嗅觉杏仁核,接收主嗅球输入并投射至下丘脑;(4)内侧杏仁核接收背侧丘脑前部输入,投射至内侧皮层、隔区和下丘脑;(5)由伏隔核与腹侧苍白球构成的腹内侧柱,投射至中央杏仁核、下丘脑和后结节;(6)吻侧构成背侧纹状体、尾侧构成背侧苍白球的侧柱,以及构成腹侧纹状体的腹外侧柱。我们得出结论:无尾类由延伸的中央杏仁核、犁鼻器杏仁核和嗅觉杏仁核组成的尾中外侧复合体代表了杏仁复合体的原始状态。在哺乳动物端脑演化过程中,该复合体向内侧移位并内卷。哺乳动物的基底外侧杏仁核显然是演化新结构,但无尾类杏仁复合体的内侧部分在输入输出方面与该结构存在相似性,可能具有类似功能。[2] 兔视网膜中的生物胞素标记广视场双极细胞在内网状层第5层具有广泛的轴突分支,其树突野虽宽阔却未接触所有视锥细胞。本研究旨在确定该细胞接触的视锥类型。我们通过生物胞素选择性摄取标记双极细胞,用花生凝集素标记视锥细胞,再采用抗蓝视锥蛋白与红绿视锥蛋白抗体鉴定各视锥类型。结果显示生物胞素标记细胞选择性接触外节被蓝视锥蛋白染色的视锥细胞,而避开未染色视锥。由此得出结论:该生物胞素标记广视场双极细胞是兔视网膜中的ON型蓝视锥双极细胞,与灵长类、小鼠和黄鼠视网膜中已报道的蓝视锥双极细胞具有同源性[3]。 |
| 动物实验 |
In Vivo Rat Experiments [1]
To test whether the newly designed tracers, L1 and L2, were more stable in vivo than conventional Biocytin (L), we performed iontophoretic injections of all three compounds into the cortex of 10 albino rats (Sprague−Dawley). Five rats (two with L, one with L1, and two with L2) were sacrificed after a survival time of 24 h, three rats (with L, L1, and L2) after a survival time of 96 h, and two rats (with L and L1) after 1 h. For the experiments, 120 specimens of the fire-bellied toad Bombina orientalis were used. The animals were taken from a breeding colony at our institute. For the reconstruction of the anatomy of the telencephalon, 15-μm-thick transverse, horizontal and sagittal sections were made, embedded in paraffin, and counterstained with Klüver-Barrera. For the Biocytin labeling experiments, animals were deeply anesthetized in 0.5% tricaine methanesulfonate, cooled to a body temperature of 5°C, and perfused transcardially with 40 ml of ice-cold oxygenated Ringer's solution consisting of Na+ 100 mM, K+ 2 mM, Ca2+ 2 mM, Mg2+ 0.5 mM, Cl− 82 mM, HCO3− 25 mM, glucose 11 mM, buffered to a final pH of 7.3 through continuous perfusion of 95% O2 + 5% CO2. Brains were removed from the skull by a ventral approach. Anterograde and retrograde labeling was made by application of Biocytin crystals to the brain. The isolated brains, either intact or longitudinally split into two halves, were exposed to the air and dried by using paper tissue, before small lesions were made with a glass micropipette at the site of application. The application sites and the number and mode of application are listed in Table 1. Ten minutes were allowed for the uptake of biocytin. Afterward, the brains were stored in Ringer's solution at room temperature for 4 hours and at 4°C for 16 to 32 hours. Brains were fixed in 2% paraformaldehyde and 2% glutaraldehyde, then 50-μm-thick transverse sections were cut on a Vibratome. Biocytin was visualized by means of an avidin-biotin-horseradish peroxidase complex using diaminobenzidine as chromogen with heavy-metal intensification (Adams, 1981). Sections were lightly counterstained with cresyl violet, dehydrated in ethanol, cleared in xylene, and cover-slipped. For intracellular labeling experiments, brains were split longitudinally, and half of the brain was fixed with stainless steel insect pins on the floor of a recording chamber (modified after Schaffer, 1982) with the cut medial surface pointing upward. The brain was continuously perfused with oxygenated Ringer's solution (6 ml/min) at a temperature of 14–18°C. The medial approach turned out to be favorable, because myelinated fibers below the lateral and ventral surfaces form a strong barrier to microelectrode penetrations. However, due to that method, possible contralateral projections of neurons could only be identified by their axons or axon collaterals entering the commissures. For intracellular labeling, micropipettes were filled with a 2% solution of Biocytin dissolved in 0.3 M potassium chloride. The impedance of the electrodes was 80–160 MΩ. The electrodes were advanced in steps of 1 or 2 μm, while a 200-msec hyperpolarizing current of 0.2 nA was applied every second. When a nerve cell membrane was penetrated, the potential dropped from −20 to −65 mV. For injection of Biocytin, a pulsed current of 1 nA was applied for 4 minutes. Usually, only one injection was done in each half of the brain. After the injection, brains were stored in oxygenated Ringer's solution at room temperature for 3 hours and at 4°C overnight. The brains were processed as described above for the anterograde and retrograde labeling experiments. Reconstructions of labeled neurons were made by hand with the aid of a camera lucida. Sections were scanned with a digital camera at a resolution of 3,900 × 3,090 pixels.[2] Wide-field bipolar cells were selectively labeled in rabbit retinas by vitreal injections of Biocytin. Adult New Zealand White rabbits were first anesthetized with an intramuscular injection of ketamine (30 – 60 mg/kg) and xylazine (10 mg/kg) and then 20 μl of 5% Biocytin in ddH2O and 0.5% DMSO were injected into each eye. After 40 – 50 hour incubations, the rabbits were reanesthetized using the same agents and the eyes were ennucleated, hemisected and the retinas removed from the eye cup while immersed in oxygenated Ames Medium. Rabbits were euthanized with Euthosol (100 mg/kg, iv). Free floating retinas were fixed for 60 – 90 minutes in 4% formaldehyde and rinsed thoroughly in three changes of sodium phosphate buffer (0.1M, pH 7.4) over 30 minutes. For retinal wholemounts, tissue was stained immediately with antibodies. For retinal sections, tissue was embedded in 4% agarose (low melting point) and cut into 60 μm sections using a vibratome. The tissue was processed to reveal Biocytin next. Retinas were incubated overnight at 4°C in 0.25M Tris buffer with 0.5% Triton-X 100, rinsed in the same buffer the next day and then incubated for two days in avidin DN.. After rinsing, the retinas were incubated overnight in fluorescein anti-avidin D followed by thorough rinsing in buffer. The final step was to label all cones with peanut agglutinin, which labels certain disaccharides on the surface of outer and inner segments of all cones and in punctuate regions at the cone pedicles. Endogenous avidin and biotin in the retina was blocked by preincubating the tissue in an avidin/biotin blocking solution for 30 minutes at room temperature, rinsed in tris buffer and then incubated with biotinolated peanut agglutinin in the microwave at 150 watts for 13 minutes. The tissue was rinsed in sodium phosphate buffer (3 × 60 sec) and the lectin revealed with Texas Red conjugated strepavidin for 10 minutes, rinsed thoroughly in phosphate buffer and coverslipped in Vectashield shortly before viewing in the microscope. Retinal sections were processed using the same procedures except for the Biocytin step; incubation in avidin DN was shortened to overnight (rather than several days) and to two hours in fluorescein anti-avidin. Imaging [3] Labeled retinas were imaged with an Olympus Fluoview 300 Confocal microscope equipped with helium, argon and neon lasers and an Olympus 60 X water immersion objective (NA 1.2) at resolutions of 1024 × 1024 or 2048 × 2048. A series of optical sections in a single channel were collected every 0.5 μm in the inner plexiform layer to show the wide-field bipolar cell axons, in the outer plexiform layer to show the contacts made by the bipolar cell dendrites with overlying cones, and through the outer nuclear layer to link individual cone pedicles with corresponding outer segments. Each channel was scanned separately to reduce spectral cross-talk between channels. Comparisons of the cones, opsins and the Biocytin labeled cells were made by merging images of like focal planes using MetaVue (v 6.1) or Adobe PhotoShop and corrected for brightness and contrast using Adobe Photoshop. |
| 参考文献 |
|
| 其他信息 |
Biocytin is a monocarboxylic acid amide that results from the formal condensation of the carboxylic acid group of biotin with the N(6)-amino group of L-lysine. It has a role as a mouse metabolite. It is an azabicycloalkane, a thiabicycloalkane, a member of ureas, a monocarboxylic acid amide, a non-proteinogenic L-alpha-amino acid and a L-lysine derivative. It is functionally related to a biotin. It is a tautomer of a Biocytin zwitterion.
Biocytin has been reported in Homo sapiens and Brassica napus with data available. In summary, the new stable tracing agents (L1 and L2) were designed and synthesized, and an assessment of their uptake and transport ability by commonly used histochemical procedures was performed. The linking of biotin and lysine moieties in both molecules were performed in such a way that the new molecules are resistant to cleavage by the action of biotinidase. The in vivo experiments performed on these synthesized derivatives of Biocytin proved that they are indeed more stable conjugates. The staining of neuronal cell bodies and fibers at the injection site and remote terminal fields were retained even at 96 h postinjection times, when commercial biocytin was nearly degraded. Anterograde or retrograde transport could be found in the ipsi- and contralateral cortex, striatum, thalamus, and further down in the brain stem, demonstrating their efficient transport along axons. Thus L1 and L2 represent an excellent alternative for conventional histological studies that would alleviate the problems caused by the endogenous degradation of the tracer molecule before the animal is sacrificed. Importantly, the molecular design of these agents could be easily diversified for use as multimodal tracers after coupling different reporter moieties. Thus, the reported molecules could serve as a potent tool for the development of agents that can be visualized with magnetic resonance imaging and thus be used for the in vivo study of brain connectivity. [1] In the present study, a combination of antero- and retrograde and intracellular Biocytin labeling was used, because both approaches have characteristic advantages and disadvantages. Anterograde and retrograde labeling give a good quantitative estimate of the connections of a given brain area, but no detailed information about the morphology of single neurons and their axonal projections. In addition, this method may lead to inadvertent lesioning of passing fibers, and neurons with very wide dendritic trees may be labeled by means of dendrites instead of axons. Intracellular injections on the other hand yield no quantitative information about the strength of projections and may be biased by differences in the accessibility and penetrability of neurons. For example, while in Bombina orientalis it was relatively easy to penetrate neurons in the medial, central, and lateral amygdala as well as in the ventral striatum and ventral pallidum by a medial approach in the longitudinally split brain, neurons in the lateral pallium, SPTA, and dorsal striatum were more difficult to label, because they could be reached only by crossing the ventricle. Labeling of septal neurons was most difficult, because the cells are rather loosely arranged and easily escape the microelectrode tip. Despite these caveats, only few major differences between the results from anterograde, retrograde and intracellular labeling were observed. Projections of the ventral–lateral pallium to the ventral–lateral septum, ventral pallidum, nucleus accumbens, hypothalamus, nucleus parabrachialis/nucleus visceralis secundarius, and medulla oblongata were revealed by retrograde and partially by anterograde labeling, but not by intracellular labeling. Anterograde labeling also revealed projections of the SPTA to the optic tectum and torus semicircularis and of the ventral–lateral pallium to the posterior tubercle, which were not found by intracellular labeling. On the other hand, intracellular injection into cells of the nucleus accumbens, but not anterograde tracing, revealed projections to the tegmentum and medulla oblongata. Some differences become visible, when anterograde and retrograde staining results are compared, because in our hands, Biocytin revealed a better anterograde than retrograde transport. For example, tracer application to the medulla resulted in fiber labeling in the accessory olfactory bulb, whereas application to the accessory olfactory bulb did not reveal retrogradely stained neurons in the medulla. The lateral pallium was not intensely studied here and deserves a more detailed analysis. Our anterograde and retrograde tracing experiments suggest that the ventral–lateral pallium is distinct from the dorsal–lateral pallium. It receives input from the main olfactory tract and, in contrast to the dorsal–lateral pallium, reveals a strong back projection to the main olfactory bulb. In the very caudal ventral portion of the ventral–lateral pallium and close to the caudal “loop” of the medial olfactory tract, a distinct cell group continuous with the adjacent caudal SPTA was labeled after application of Biocytin to the hypothalamus (cf. Figs. 6, 7). The rostrally adjacent column constituted by the ventral–lateral pallium and SPTA only revealed scattered retrogradely labeled neurons. Based on its probable input from the main olfactory bulb and its projection to the hypothalamus, we believe that at least the caudally labeled cluster of neurons are homologous to the cortical or “olfactory” amygdala of mammals, as was already suggested by Scalia et al. (1991). [2] The Biocytin stained wide-field bipolar cell is the rabbit's equivalent of the mammalian ON blue cone bipolar cell that has been described in monkey, mouse and ground squirrel retinas (Kouyama and Marshak, 1992; Haverkamp et al., 2005; Li and DeVries, 2006). Like its mammalian counterparts, the rabbit's wide-field cell is more than twice as large as any other bipolar cell type (MacNeil et al., 2004). Its axon resides in layer 5 of the the inner plexiform layer and is thus presumed to be an ON bipolar cell. Moreover, the dendrites of the wide-field cell extend throughout the inner plexiform layer and make selective contacts with cones that express blue cone opsin in their outer segments and avoid cones that do not. We assume that these points of contact represent synaptic junctions because we were able to follow the dendrites in through focus series into blue cone pedicles and see them terminate adjacent to patches of PNA labeling. Invaginating bipolar processes are a hallmark of ON cone bipolar cells and mark the locations where synapses between bipolar cells and cones occur (Hopkins and Boycott, 1995). A confocal study of the cone pedicle has shown that PNA labeling overlaps that of bassoon-immunoreactive ribbons and is in register with Goα, a marker for ON-bipolar cells (Haverkamp et al., 2001). This suggests that the proximity of the PNA label with the Biocytin stained processes in the blue cone pedicles reflects a synaptic relationship. Synaptic communication between blue cone bipolar cells and blue cones has been clearly shown by Li and DeVries (2006) in ground squirrel retina. They demonstrated that only one type of bipolar cell made exclusive contacts with blue cones and these contacts were functional; depolarization in a presynaptic cone generated a prominent outward current in a coupled bipolar cell. [2] In addition to the contacts that the wide-field cell dendrites made with cones, the dendrites also included thin processes (Famiglietti, 1981) that merged with the thin dendrites of adjacent cells (Jeon and Masland, 1995). These processes usually arose from a terminal cluster aligned with a blue cone and were smooth and not associated with any cone pedicles that might suggest a synaptic junction. It is likely that gap junctions exist between the thin dendrites of adjacent cells, but their existence could not be established in the confocal images. [2] One significant difference observed in the cone contacts of the rabbit wide-field bipolar cell was the degree of divergence of the photoreceptor input. In primates and mice, the density ratio of blue cone bipolar cells to blue cones is approximately 1:2 and each blue cone is contacted by more than one blue cone bipolar cell (Kouyama and Marshak, 1992; Kouyama and Marshak, 1997; Schein et al., 2004; Haverkamp et al., 2005). In rabbits, the divergence of blue cone input onto each wide-field bipolar cell depends on retinal eccentricity. In dorsal retina, where the density of blue cones is low relative to the numbers of wide-field bipolar cells, blue cones were contacted by dendrites from more than one wide-field cell. In ventral retina, multiple cones were more likely to converge onto each wide-field bipolar cell with none of them contacting more than one (Figure 7). The advantage of this arrangement could be to preserve spatial representation of the blue signal in ventral retina while allowing for better detection of the blue-cone signal in dorsal retina where the number of blue cones is lowest. [2] Dual pigment cones [2] A small fraction of cones in peripheral ventral rabbit retina were labeled with antibodies against both blue and red-green cone opsins. Mixed opsin cones are common in many mammalian retinas (Szél et al., 2000; Lukáts et al., 2005), but their specific function is not known. In mouse retina, the majority of cones express dual cone opsins (Applebury et al., 2000), but blue cone bipolar cells still make appropriate connections with genuine blue cones (Haverkamp et al., 2005) suggesting there exists a chromatically segregated blue channel in mouse retina. In the rabbit retina, the Biocytin wide-field cells contacted all blue cones in their dendritic field except in the ventral rim where mixed pigment cones were observed. At this eccentricity, the cell dendrites contacted a subset of blue cones suggesting that cones expressing more than one cone opsin are functionally different from genuine blue cones. The advantage of having such mixed cones is not yet understood, but may be an adaptation of diurnal mammals for better detection of predators against a blue sky background (Ahnelt and Kolb, 2000; Peichl, 2005). [2] Function of wide-field bipolar cells [2] The blue cone selectivity of the Biocytin wide-field bipolar cell and its structural similarity to blue cone bipolar cells in monkey, mouse and ground squirrel retinas suggests that it is involved with color processing in rabbit retina. The idea that wide-field cells would be selective for blue cones was first proposed by Famiglietti (1981). From counts of cells and measurements taken between terminal clusters on the wide-field bipolar dendrites, Famiglietti predicted that if the wide-field cells were selective for blue cones, one should see an increase in blue cone density toward the periphery. This prediction was born out by cone labeling studies showing a general increase in blue cone density toward the ventral periphery (Juliusson et al., 1994; Famiglietti and Sharpe, 1995) and a tendency for wide-field cells in ventral retina to contact more blue cones with increased distance from the visual streak. The ability of wide-field cells to make selective contacts with blue cones would allow them to deliver a spectrally segregated signal to ganglion cells that stratify in layer 5 of the inner plexiform layer. [2] There is still some question concerning the identity of OFF-type blue cone bipolar cells in the rabbit retina. Famiglietti (1981) described two populations of wide-field bipolar cells in Golgi-stained tissue: one that stratified in sublamina b (type wb), which is most likely the Biocytin wide-field bipolar cell and a similar type that stratified in sublamina a (type wa). Wa cells possessed “extensive” axons of unspecified length and a narrow dendritic arbor that comprised between 1 and 4 primary dendrites. Each dendrite contacted a cone, including some cones that were shared with wb cells. So far, other studies of rabbit bipolar cells have not found an equivalent type (Mills and Massey, 1992; McGillem and Dacheux, 2001; MacNeil et al., 2004). The largest bipolar cell identified that stratified in sublamina a was type DAPI-Ba3 (Mills and Massey, 1992), but the dendrites were much more extensive than those of wa cells, and contacted an average of 16 cones per cell. Chiao and Liu (2006) injected a pair of OFF bipolar cells in dorsal retina that appeared to contact blue cones exclusively. The cells had narrow dendritic fields with four primary dendrites (like those of wa cells) and each dendritic terminal was aligned with blue cone opsin staining. However, the axon field diameters of these cells were relatively small compared with the axons of Biocytin wide-field bipolar cells (73 μm versus >200 μm in diameter), so it is unlikely that Chiao and Liu's cells are equivalent to type wa either. A more thorough examination of OFF bipolar cell types will be needed to clarify the morphology of the OFF blue cone bipolar cell population. [2] Circuitry of the Biocytin wide-field bipolar cell [2] Recordings of ganglion cells in rabbit retina have revealed the presence of blue ON/green OFF, color opponent ganglion cells (Caldwell and Daw, 1978; De Monasterio, 1978; Vaney et al., 1981). The circuitry underlying these light responses is not yet known but could be formed by the selective wiring of ON and OFF cone bipolar cells onto a bistratified ganglion cell, as has been shown in primates (Dacey and Lee, 1994; Calkins et al., 1998). A good candidate for such wiring is the G3 ganglion cell in rabbit retina (Rockhill et al., 2002). It has a bistratified dendritic arbor with the inner dendrites located at the border between strata 4 and 5, in the vicinity of the Biocytin wide-field cell axons, and an outer arbor located in stratum 2. We have shown that the dendrites of the wide-field bipolar cell selectively contact blue cones in the outer plexiform layer and its axon stratifies in layer 5 of the inner plexiform layer. With combined input from red-green cones carried by an OFF bipolar cell that stratifies in sublamina A of the inner plexiform layer, the G3 ganglion cell could be homologous to the blue–yellow cell described in primates (Calkins et al., 1998; Dacey, 2000). Alternatively, the wide-field bipolar cell could synapse with a monostratified ganglion cell that stratifies in layer 5, such as the Ib2 (Famiglietti, 2004) or G10 ganglion cell types (Rockhill et al., 2002). In recent recordings of blue cones in primate retina, Packer and colleagues have shown that blue-yellow spectral opponency originates within the blue cones themselves (Packer et al., 2007). Therefore, a bistratified ganglion cell would not be a prerequisite for generating the types of color opponent responses that have been recorded in rabbit retinal ganglion cells and the responses could be carried to the brain by a monostratified ganglion cell instead.[3] |
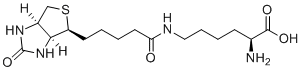
| 分子式 |
C16H28N4O4S
|
|---|---|
| 分子量 |
372.48
|
| 精确质量 |
372.183
|
| 元素分析 |
C, 51.59; H, 7.58; N, 15.04; O, 17.18; S, 8.61
|
| CAS号 |
576-19-2
|
| 相关CAS号 |
576-19-2;
|
| PubChem CID |
83814
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
748.0±60.0 °C at 760 mmHg
|
| 熔点 |
-245ºC (dec.)
|
| 闪点 |
406.2±32.9 °C
|
| 蒸汽压 |
0.0±5.4 mmHg at 25°C
|
| 折射率 |
1.548
|
| LogP |
-1.02
|
| tPSA |
158.85
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
491
|
| 定义原子立体中心数目 |
4
|
| SMILES |
S1C([H])([H])[C@@]2([H])[C@@]([H])([C@]1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[C@@]([H])(C(=O)O[H])N([H])[H])=O)N([H])C(N2[H])=O
|
| InChi Key |
BAQMYDQNMFBZNA-MNXVOIDGSA-N
|
| InChi Code |
InChI=1S/C16H28N4O4S/c17-10(15(22)23)5-3-4-8-18-13(21)7-2-1-6-12-14-11(9-25-12)19-16(24)20-14/h10-12,14H,1-9,17H2,(H,18,21)(H,22,23)(H2,19,20,24)/t10-,11-,12-,14-/m0/s1
|
| 化学名 |
N6-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoyl)-L-lysine
|
| 别名 |
Biotinyl-L-lysine; Biocytin; 576-19-2; N'-Biotinyl-L-lysine; H-Lys(biotinyl)-OH; Biotinyl-L-lysine; epsilon-N-Biotinyl-L-lysine; Ne-Biotynyl-L-lysine; N-biotinyl-L-lysine; Biocytin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~50 mg/mL (~134.24 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (268.47 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6847 mL | 13.4235 mL | 26.8471 mL | |
| 5 mM | 0.5369 mL | 2.6847 mL | 5.3694 mL | |
| 10 mM | 0.2685 mL | 1.3424 mL | 2.6847 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|