| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
This research examined the distribution of low dietary doses of bisphenol A (BPA). When female rats received 50 ug/kg (14)C-BPA orally, radioactivity was distributed throughout the body, with especial presence in the uterus. Pre-treatment with estradiol or the estrogen antagonist ICI 182,780 significantly reduced radioactivity in the uterus. The majority of BPA at the uterus was determined to be aglycone (receptor-active) via GC-MS. Subsequently, mice given 0.5, 5, or 50 ug/kg (14)C-BPA showed more radioactivity in the uterus than in other non-metabolic tissues. When female mice received 1, 7, or 28 daily doses of 50 ug/kg (14)C-BPA, then were measured 24 hr after the last dose, significantly more radioactivity was detected in the uterus, liver, and kidney following repeated doses. Collectively, these data provide evidence for the in vivo interaction of BPA with estrogen receptors. They also indicate elevated presence of BPA in reproductive tissues after repeated low doses. The widespread human exposure to Bisphenol A (BPA), an endocrine disruptor interfering with developmental processes, raises the question of the risk for human health of BPA fetal exposure. In humans, highly variable BPA concentrations have been reported in the feto-placental compartment. However the human fetal exposure to BPA still remains unclear. The aim of the study was to characterize placental exchanges of BPA and its main metabolite, Bisphenol A-Glucuronide (BPA-G) using the non-recirculating dual human placental perfusion. This high placental bidirectional permeability to the lipid soluble BPA strongly suggests a transport by passive diffusion in both materno-to-fetal and feto-to-maternal direction, leading to a calculated ratio between fetal and maternal free BPA concentrations of about 1. In contrast, BPA-G has limited placental permeability, particularly in the materno-to-fetal direction. Thus the fetal exposure to BPA conjugates could be explained mainly by its limited capacity to extrude BPA-G. When administered as a single dose by gavage to male CFE rats, 28% of the (14)C-labeled bisphenol A was excreted in the urine (primarily as the glucosamide) and 56% in the feces (20% as free bisphenol A, 20% as a hydroxylated bisphenol A, and the rest as an unidentified conjugate). No carbon-labeled residues were detected in animals killed after 8 days. Toxicokinetics of radiolabeled (14C) bisphenol A was studied in the common frog (Rana temporaria) at two experimental temperatures (7 and 19 °C). The growth rate of the tadpoles during the 96-hr experiment was very slow at 7 °C, but the weight of tadpoles almost tripled at 19 °C. At all tested exposure concentrations (0.2, 1.5, 10, and 100 ug/L), conditional uptake rate constants (ku) were 69 to 82%, and elimination rates (ke) 79 to 90% lower, at 7 °C than at 19 °C. On the contrary, bioconcentration factors (BCFs) were higher at 7 °C than at 19 °C. Total accumulated bisphenol A per individual was higher at 19 °C, which is in agreement with higher ku at 19 °C. Exposure concentrations did not have any constant effect on BCFs at the two temperatures. The results of the current experiment suggest that higher temperature increases uptake and total amount of chemical in frog tadpoles but does not necessarily lead to higher BCFs. High temperature may have increased the growth rate more than the uptake rate, resulting in a net dilution of bisphenol A in tadpole tissues. The observed difference in BCFs also could be a result of temperature-induced changes in allometric relationships (increased surface area to volume ratio) and/or more effective elimination in more developed tadpoles at high temperature. For more Absorption, Distribution and Excretion (Complete) data for Bisphenol A (27 total), please visit the HSDB record page. Metabolism / Metabolites ... The metabolism of bisphenol A [2,2-bis(4-hydroxyphenyl)propane] (BPA) by CD1 mice liver microsomal and S9 fractions was investigated. Nine metabolites were isolated and characterized using HPLC and mass spectrometry. Many of these metabolites were characterized for the first time in mammals, namely isopropyl-hydroxyphenol (produced by the cleavage of BPA), a bisphenol A glutathione conjugate, glutathionyl-phenol, glutathionyl 4-isopropylphenol, and BPA dimers. ... In this study, bisphenol A (BPA) levels in 30 healthy Koreans (men, N=15, 42.6 +/- 2.4 years; women, N=15, 43.0 +/- 2.7 years) were analyzed from urine treated with/without beta-glucuronidase and/or sulfatase by an RP-HPLC with fluorescence detection. The total BPA concentrations including free BPA and the urinary conjugates were similar in men and women (2.82 +/- 0.73 and 2.76 +/- 0.54 ng/mL, respectively), but gender differences were found in the levels of urinary BPA conjugates. Men had significantly higher levels of BPA-glucuronide (2.34 +/- 0.85 ng/mL) than women (1.00 +/- 0.34 ng/mL), whereas women had higher levels of BPA-sulfate (1.20 +/- 0.32 ng/mL) than men (0.49 +/- 0.27 ng/mL). ... The objective of this study was to determine if a route dependency exists in the pharmacokinetics and metabolism of (14)C-labeled bisphenol A (BPA) following single oral (po), intraperitoneal (ip), or subcutaneous (sc) doses of either 10 or 100 mg/kg to Fischer 344 rats. Results indicated a marked route dependency in the pharmacokinetics of BPA. The relative bioavailability of BPA and plasma radioactivity was markedly lower following oral administration as compared to sc or ip administration. The major fraction of plasma radioactivity following oral dosing was the monoglucuronide conjugate of BPA (68-100% of plasma radioactivity). BPA was the major component in plasma at Cmax following sc or ip administration exceeded only by BPA-monoglucuronide in females dosed ip. Up to four additional unidentified metabolites were present only in the plasma of animals dosed ip or sc. One of these, found only following ip administration, was tentatively identified as the monosulfate conjugate of BPA. The monoglucuronide conjugate was the major urinary metabolite; unchanged BPA was the principal component excreted in feces. ... Previous studies demonstrated the rapid clearance of bisphenol A (BPA) from blood following oral administration to adult rats with the principal metabolite being BPA-monoglucuronide (BPA-glucuronide). Since the ontogeny of glucuronyl transferases (GT) differs with age, the pharmacokinetics of BPA were studied in neonatal animals. (14)C-BPA was administered via gavage at 1 or 10 mg/kg body weight to rats at postnatal day (pnd) 4, pnd 7, pnd 21, or to 11 week old adult rats (10 mg/kg dose only). Blood (neonates and adults) and selected tissues (neonates) were collected at 0.25, 0.75, 1.5, 3, 6, 12, 18, and 24 hr postdosing. BPA and BPA-glucuronide in the plasma were quantified by high-performance liquid chromatography; radioactivity in the plasma and tissues was quantified by liquid scintillation spectrometry. The data indicate that neonatal rats at all three ages metabolized BPA to BPA-glucuronide, although an age dependency in the number and concentration of plasma metabolites was observed, consistent with the ontogeny of GT. BPA-glucuronide and BPA concentrations in the plasma were greater in neonates than in adults, except at 24 hr postdosing, suggesting an immaturity in the development of hepatic excretory function in neonatal rats. ... A dose dependency in the metabolism and pharmacokinetics of BPA administered to neonates was also observed with nearly complete metabolism of BPA to BPA-glucuronide (94-100% of the plasma radioactivity) at a dose of 1 mg/kg. This was in contrast to finding up to 13 different plasma metabolites observed at the 10 mg/kg dose. ... For more Metabolism/Metabolites (Complete) data for Bisphenol A (8 total), please visit the HSDB record page. Bisphenol A has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[4-[2-(4-hydroxyphenyl)propan-2-yl]phenoxy]oxane-2-carboxylic acid. Bisphenol A (BPA) is rapidly absorbed from the gastrointestinal tract after ingestionand is then converted to a number of metabolites, mainly BPA glucuronide, in the liver . Metabloites of Bsiphenol A (BPA) include isopropyl-hydroxyphenol (produced by the cleavage of BPA), a bisphenol A glutathione conjugate, glutathionyl-phenol, glutathionyl 4-isopropylphenol, and BPA dimers. The monoglucuronide conjugate was the major urinary metabolite; unchanged BPA is the principal component excreted in feces (A287, A288). Biological Half-Life ... Following a single oral or intravenous (iv) dose of 100 ug/kg (ring-(14)C(U)) radiolabeled bisphenol A ((14)C-BPA) to male and female cynomolgus monkeys ... the terminal elimination half-life was larger post-iv dose (t(1/2iv) = 13.5 to 14.7 hr) than post-oral dose (t(1/2oral) = 9.63 to 9.80 hr). After iv dose, the fast-phase half-life (t(1/2f)) of total radioactivity was 0.61 to 0.67 hr. The t(1/2f) of unchanged (14)C-BPA for females (0.39 hr) was smaller than that for males (0.57 hr). ...The oral bioavailability was determined after administration of relatively low iv (0.1 mg/kg) and oral (10 mg/kg) doses of bisphenol A to rats. ... The apparent terminal elimination half-life of bisphenol A (21.3 +/- 7.4 hr) after oral administration was significantly longer than that after iv injection ... Bisphenol A was injected intravenously to mouse, rat, rabbit and dog (1-2 mg/kg doses). The obtained serum concentration-time profiles were best described by bi-exponential equations in all these animal species, with the mean ... t(1/2) of 39.9 min in mouse, 37.6 min in rat, and 40.8 min in rabbit, and 43.7 min in dog, respectively. ... The simple allometric scaling and different time transformation methods predicted the human t(1/2) ranging from 43.6 to 196.2 min. ... The toxicokinetics of bisphenol A (BPA) in F344 rats, cynomolgus monkeys and chimpanzees /were examined/. ...After the oral administration of 10 mg/kg BPA, both C(max) and AUCs of BPA metabolites were ranked in the following order: cynomolgus monkeys>chimpanzees>rats, and the terminal elimination half-life (T(1/2)) in rats was greater than that in cynomolgus monkeys and chimpanzees, suggesting the enterohepatic circulation of BPA in rats. ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Bishphenol A (BPA) is a solid. It is used in the manufacture of epoxy resins and polycarbonates for food packaging. HUMAN STUDIES: Allergic contact dermatitis caused by polyvinyl chloride gloves is rarely reported, and in only 2 cases was BPA considered to be the responsible sensitizer. A clinical report describes photoallergic contact dermatitis to BPA in a group of eight outdoor workers. Urinary BPA may be associated with declined semen quality and increased sperm DNA damage. A correlation was observed between environmental exposure to BPA and the genesis of fetal malformations. Maternal conjugated BPA was also associated with a higher risk of aneuploid and euploid miscarriage. BPA is an endocrine disruptor with estrogenic properties that can adversely affect meiotic spindle assemblies. Data indicate that BPA exposure in female patients may interfere with oocyte quality during in vitro fertilization (IVF). Male BPA exposure may affect embryo quality during IVF. BPA was found to be cytotoxic, but not genotoxic in human cell lines. ANIMAL STUDIES: BPA caused serious damage to the eyes of rabbits, but demonstrated negligible skin irritation potential in rabbits. The effect of BPA on fertility was evaluated in an extensive oral two generation reproduction toxicity study in rats. No clinical signs of toxicity or effects on body weight gain during lactation were observed in F1 and F2 pups. No treatment-related changes were seen in the litter size, survival, sex ratio, anogenital distance and reflex ontogeny. BPA significantly accelerated mammary tumorigenesis in a transgenic mouse model that spontaneously develops tumors. In mice, exposure to a low dose of BPA, only during gestation, had immediate and long-lasting, transgenerational effects on mRNA in brain and social behaviors. Prenatal exposure to BPA mainly affected male rats and abolished sex differences in rearing behavior in the open-field test and struggling behavior in the forced swimming test. In mice, prenatal exposure to BPA affected pituitary gonadotroph development in females. BPA was not mutagenic using tester strains of Salmonella typhimurium (TA 98, TA 100 and TA 102) in the presence and absence of metabolic activation. However, in vivo BPA exposure in rats caused a significant increase in the frequency of micronucleus in polychromatic erythrocytes, structural chromosome aberrations in bone marrow cells and DNA damage in blood lymphocytes. Exposure to BPA in rodents was shown to induce obesity. Furthermore, feeding Drosophila melanogaster males with BPA significantly inhibited the expression of insulin-like peptides. ECOTOXICITY STUDIES: BPA demonstrated sex reversal effects on the gonads in F1 Japanese quail (Coturnix japonica) embryos. Vitellogenin induction in rainbow trout described after intraperitoneal injection of BPA. Japanese medaka (Oryzias latipes) was exposed to BPA at the sublethal concentrations of 2.28, 13.0, 71.2, 355, and 1,820 ug/L in the early life stage from fertilized eggs to 60-days posthatch. When observed for their external secondary sex characteristics, no males were identified in the 1,820-ug/L treatment. In addition, histological examination showed that 32% of fish in the 1,820-ug/L group had testis-ova composed of both testicular germ cells and oocytes. The effect of BPA on gene expression in Arabidopsis thaliana was determined using microarray analysis and quantitative gene PCR. Many hormone responsive genes showed changes in expression after BPA treatment. BPA disrupted flowering by a mechanism that may involve disruption of auxin signaling. Bisphenol is an endocrine disruptor. Low doses of bisphenol A can mimic the body's own hormones, possibly causing negative health effects. There is thus concern that long term low dose exposure to bisphenol A may induce chronic toxicity in humans (L705). Toxicity Data LC (mice) > 1,700 mg/m3/2h; (inhalation) LD50: 2230 mg/kg (Oral, Rabbit) (T249) Interactions We investigated the possible transmission of heritable changes via the sperm, following preconceptional exposure of mice to bisphenol A (BPA), either alone or in combination with X-irradiation. Males were exposed for 8 weeks to BPA, X-rays or both agents, and mated to unexposed females. Pre- and postnatal development of the offspring of exposed males was examined. Both BPA alone and the combined exposure slightly affected postnatal development. Combined exposure induced two-fold higher postnatal mortality than BPA the alone, whereas BPA exposure caused reduced body weight and diminished sperm quality in F1 generation. This study was designed to investigate the effects of 2 weeks of exposure of male mice to bisphenol A (BPA) alone or in a combination with X-rays on the sperm count and quality as well as induction of DNA strand breaks in somatic and germ cells. Pzh:SFIS male mice were exposed to X-rays (0.05 and 0.10 Gy) or BPA (5, 10, 20, and 40 mg/kg) or to a combination of both (0.05 Gy + 5 mg/kg body weight of BPA and 0.10 Gy + 10 mg/kg of BPA). Both X-rays and BPA administered alone decreased sperm count and quality. X-rays induced DNA strand breaks in spleen cells, whereas BPA induced DNA strand breaks in lymphocytes and in cells from spleen, kidneys, and lung and in germ cells. After combined exposure to both agents, sperm count and quality were similar as after exposure to each agent alone and significantly reduced, compared to control. Levels of DNA damage in somatic and germ cells after combined exposure to lower, as well as higher, doses were significantly reduced, compared to the effects of BPA alone. Results confirmed the mutagenic ability of BPA. Combined exposure to X-rays and BPA leads to the prevention of DNA damage in somatic and germ cells of mice. Bisphenol A (BPA) is employed in the manufacturing of epoxy, polyester-styrene, and polycarbonate resins, which are used for the production of baby and water bottles and reusable containers, food and beverage packing, dental fillings and sealants. The study was designed to examine the effects of 8-week exposure (a full cycle of spermatogenesis) to BPA alone and in a combination with X-irradiation on the reproductive organs and germ cells of adult and pubescent male mice. Pzh:Sfis male mice were exposed to BPA (5, 10, and 20 mg/kg) or X-rays (0.05 Gy) or to a combination of both (0.05 Gy + 5 mg/kg bw BPA). The following parameters were examined: sperm count, sperm motility, sperm morphology, and DNA damage in male gametes. Both BPA and X-rays alone diminished sperm quality. BPA exposure significantly reduced sperm count in pubescent males compared to adult mice, with degenerative changes detected in seminiferous epithelium. This may suggest a higher susceptibility of germ cells of younger males to BPA action. Combined BPA with X-ray treatment enhanced the harmful effect induced by BPA alone in male germ cells of adult males, whereas low-dose irradiation showed sometimes protective or additive effects in pubescent mice. ... The aim of the present study was to investigate if bisphenol A induces oxidative stress in the liver of rats and if co-administration of vitamin C, an antioxidant, can prevent oxidative stress. Bisphenol A (0.2, 2.0 and 20 ug/kg body weight per day) and bisphenol A + vitamin C (0.2, 2.0, 20 ug + 40 mg/kg body weight per day) was orally administered to rats for 30 days. After 24 hr of the last treatment, rats were killed using overdose of anesthetic ether. Body weights of the animals and the weights of liver showed no significant changes. The activities of antioxidant enzymes, superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase were decreased in mitochondrial and microsome-rich fractions of liver. The levels of hydrogen peroxide and lipid peroxidation increased in the treated rats when compared with the corresponding group of control animals. Activity of alanine transaminase, a marker enzyme of hepatic injury remained unchanged in the treated rats as compared with the corresponding control rats. Co-administration of bisphenol A and vitamin C showed no changes in the activities of superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase and in the levels of hydrogen peroxide and lipid peroxidation as compared with the corresponding control groups. For more Interactions (Complete) data for Bisphenol A (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat (male, F344) oral 4100 mg/kg LD50 Rat (female, F344) oral 3300 mg/kg LD50 Mouse (male) oral 5280 mg/kg LD50 Mouse female (B6C3F1) oral 4100 mg/kg For more Non-Human Toxicity Values (Complete) data for Bisphenol A (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Bisphenol A (BPA) can cause developmental toxicity according to The National Toxicology Program's Center for the Evaluation of Risks to Human Reproduction. It can cause female reproductive toxicity according to an independent committee of scientific and health experts.
4,4'-isopropylidenediphenol appears as white to light brown flakes or powder. Has a weak medicine odor. Sinks in water. (USCG, 1999) Bisphenol A is a bisphenol that is 4,4'-methanediyldiphenol in which the methylene hydrogens are replaced by two methyl groups. It has a role as a xenoestrogen, an environmental contaminant, a xenobiotic and an endocrine disruptor. Bisphenol A is a diphenylmethane derivative with two hydroxyphenyl groups. Bisphenol A (BPA) is a colorless solid that is used in the synthesis of commercial plastics, including polycarbonates and epoxy resins, which are incorporated into a wide variety of consumer goods. Ingested BPA may exhibit estrogenic effects. Exposure to BPA may increase the risk of certain cancers. Bisphenol A, commonly abbreviated as BPA, is an organic compound with two phenol functional groups. It is a difunctional building block of several important plastics and plastic additives. With an annual production of 2-3 million metric tonnes, it is an important monomer in the production of polycarbonate. It is a potential food contaminant arising from its use in reusable polycarbonate food containers such as water carboys, baby bottles and kitchen utensils. Suspected of being hazardous to humans since the 1930s, concerns about the use of bisphenol A in consumer products were regularly reported in the news media in 2008 after several governments issued reports questioning its safety, and some retailers removed baby bottles and other children's products made from it from their shelves. See also: Anoxomer (monomer of); Polycarbonate (annotation moved to) ... View More ... Mechanism of Action This study evaluated the effects of bisphenol A (BPA) on human endometrial stromal fibroblast (ESF) differentiation and expression of genes involved in estrogen metabolism. Human ESF from eight hysterectomy specimens were cultured and treated with 5-100 umol/L of BPA + or - estradiol or 8-br-cAMP for 48 hr. mRNA expression was analysed by real-time reverse-transcription PCR. 8-br-cAMP-induced human ESF decidualization was confirmed by expression of insulin-like growth factor binding protein-1 (IGFBP1) and prolactin secretion. Short-term exposure (48 hr) decreased human ESF proliferation (P<0.04) not due to apoptosis. High doses of BPA significantly induced IGFBP1 mRNA and protein, decreased P450scc mRNA, reversed the 8-br-cAMP-induced increase in HSD17B2 (estradiol to estrone conversion) in a dose-dependent manner and down-regulated HSD17B1 expression (oestrone to estradiol conversion; P = 0.03). 8-br-cAMP significantly potentiated this effect (P=0.028). BPA had no significant effect on aromatase and PPAR gamma expression. The estrogen-receptor antagonist ICI had no effect on gene expression in BPA-treated cells, and estrogen receptor a, but not estrogen receptor beta, was significantly down-regulated by high doses of BPA (P=0.028). BPA has an endocrine-disrupting effect on human ESF function and gene expression but the underlying mechanisms appear not to involve estrogen-mediated pathways. |
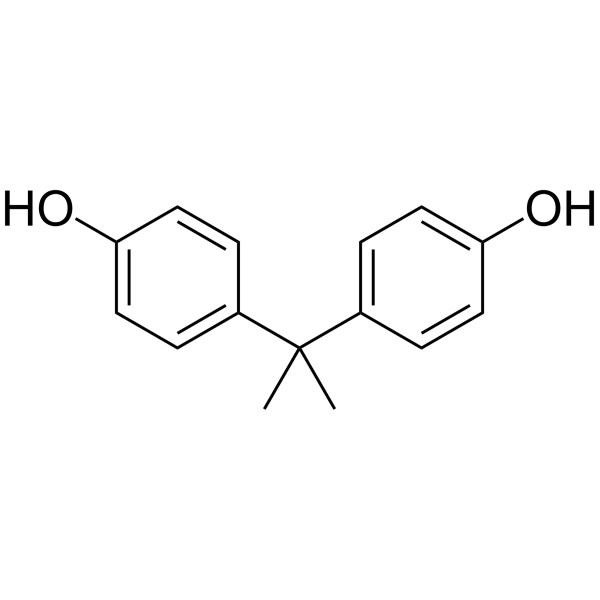
| 分子式 |
C₁₅H₁₆O₂
|
|---|---|
| 分子量 |
228.29
|
| 精确质量 |
228.115
|
| CAS号 |
80-05-7
|
| 相关CAS号 |
27100-33-0;25766-59-0;2444-90-8 (di-hydrochloride salt)
|
| PubChem CID |
6623
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
400.8±25.0 °C at 760 mmHg
|
| 熔点 |
158-159 °C(lit.)
|
| 闪点 |
192.4±17.8 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.599
|
| LogP |
3.43
|
| tPSA |
40.46
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
209
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
IISBACLAFKSPIT-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3
|
| 化学名 |
4-[2-(4-hydroxyphenyl)propan-2-yl]phenol
|
| 别名 |
Bisphenol A; Bisphenol A
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~438.04 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (9.11 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (9.11 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (9.11 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3804 mL | 21.9020 mL | 43.8039 mL | |
| 5 mM | 0.8761 mL | 4.3804 mL | 8.7608 mL | |
| 10 mM | 0.4380 mL | 2.1902 mL | 4.3804 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。