| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
DNA synthesis; antimetabolite
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:在 RG2 大鼠神经胶质瘤细胞中,溴脱氧尿苷可诱导对癌细胞系和癌症干细胞群扩张的渐进性、剂量反应性抑制。在 H9 细胞和 BJ 成纤维细胞中,溴脱氧尿苷改变细胞周期特征。 BrdU 稳定整合到 DNA 中,因此可用于评估细胞增殖和其他细胞加工。细胞测定:培养物最初以 2000 个细胞/cm2 铺板,并使用 Z2 Coulter 计数器进行定量。 RG2 大鼠神经胶质瘤细胞用 0、1、10 或 50 µM BrdU 处理 24 小时一次,并获得 18 天的累积生长曲线。处理后第 5、12 和 18 天,对对照细胞和处理细胞进行定量并以相同密度重新铺板。
|
||
| 体内研究 (In Vivo) |
在大鼠神经胶质瘤 RG2 肿瘤模型中,溴脱氧尿苷(300 mg/kg,腹膜内注射或 0.8 mg/ml,口服)可显着减缓肿瘤进展。
|
||
| 酶活实验 |
端粒长度[1]
为了确定BrdU的作用是否与端粒长度的变化有关,我们进行了TeloTAGGG测定。简言之,分离基因组DNA并用Hinf1和Rsa1酶消化。消化后,通过凝胶电泳分离DNA片段,并将其转移到尼龙膜上进行Southern印迹分析。将印迹的DNA片段与地高辛(DIG)标记的端粒重复序列特异性探针杂交,并与共价偶联至碱性磷酸酶的DIG特异性抗体孵育。最后,利用碱性磷酸酶代谢CDP-Star(一种高灵敏度的化学发光底物)对固定化端粒探针进行了可视化。 端粒酶活性[1] 我们使用TRAPeze ELISA试剂盒测定来测定我们的对照和BrdU处理的细胞中的端粒酶活性水平。简单地说,样品细胞的端粒酶在生物素化的端粒酶底物寡核苷酸(b-TS)的3′端添加了许多端粒重复序列(GGTTAG),然后通过聚合酶链式反应扩增延伸产物。用生物素化引物和DNP标记的dCTP进行延伸/扩增。因此,端粒重复扩增方案(TRAP)产物用生物素和DNP残基标记,并且标记的产物可以通过生物素-链亲和素相互作用固定在链亲和素包被的微量滴定板上,然后通过与辣根过氧化物酶(HRP)缀合的抗DNP抗体检测。TRAP产物的量通过使用底物TMB的HRP活性和随后的显色来确定。 |
||
| 细胞实验 |
最初以 2000 个细胞/cm2 铺板,使用 Z2 Coulter 计数器测量培养物。用 0、1、10 或 50 µM BrdU 处理 RG2 大鼠神经胶质瘤细胞一次 24 小时后,测量 18 天的累积生长曲线。处理五天、十二天和十八天后,对对照细胞和处理细胞进行计数并以相等的密度重新铺板。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Twelve patients were treated with continuous intravenous (24-hour) infusions of bromodeoxyuridine (BUdR) at 650 or 1,000 mg/sq m/d for up to two weeks. ... Pharmacology studies revealed a steady-state arterial plasma level of 6 X 10-7 mol/L and 1 X 10-6 mol/L during infusion of 650 and 1,000 mg/sq m/d, respectively. In vivo BUdR uptake into normal bone marrow was evaluated in two patients by comparison of preinfusion and postinfusion in vitro radiation survival curves of marrow CFUc with enhancement ratios (D0-pre/D0-post) of 1.8 (with 650 mg/sq m/d) and 2.5 (with 1,000 mg/sq m/d). In vivo BUdR incorporation into normal skin and tumor cells using an anti-BUdR monoclonal antibody and immunohistochemistry was demonstrated in biopsies from three patients revealing substantially less cellular incorporation into normal skin (less than 10%) compared with tumor (up to 50% to 70%). BrdU is absorbed from the gastrointestinal tract following parenteral injection and is presumably absorbed transplacentally (because of its teratogenic effects). Distribution and pharmacokinetics: Intra-arterial injection of BrdU into rodents results in extensive degradation... . Most of the portion which is not so degraded is incorporated into DNA of various tissues, particularly the colon, stomach, bone marrow, and spleen. The label of intraperitoneally injected deuterated BrdU in pregnant mice is also found in the liver of both mothers and embryos. BrdU tablets were implanted subcutaneously in rats, and BrdU concentrations were determined in the serum. Within 5 hr peak concentrations of 10 ug BrdU/mL blood were reached. ... With the use of agar-coated tablets, BrdU concentrations in the blood were reduced by half, and no peak concentration was found. ... Metabolism / Metabolites BrdU is degraded at a fairly rapid rate in mice and rats upon injection, in at least two metabolic pathways; one is hydrolysis at the glycosyl bond to yield bromouracil and 2-deoxyribose which is presumably then further metabolized. The other is debromination which is evidenced by liberation of bromide ion. The further fate of the remainder of the molecule has not been investigated 5-bromodeoxyuridine is phosphorylated by thymidine kinase to produce 5-bromodeoxyuridine-phosphate. (L626) |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
5-bromodeoxyuridine acts on DNA. It induces a random DNA point mutation via base substitution. The base pair will change from an A-T to a G-C or from a G-C to an A-T after a number of replication cycles. As a thymine analog, 5-bromodeoxyuridine normally pairs with adenine. Toxicity Summary 5-bromodeoxyuridine acts on DNA. It induces a random DNA point mutation via base substitution. The base pair will change from an A-T to a G-C or from a G-C to an A-T after a number of replication cycles. As a thymine analog, 5-bromodeoxyuridine normally pairs with adenine. Health Effects 5-bromodeoxyuridine is a mutagen (causes mutations), a cytotoxin, a teratogen and a weak carcinogen. The primary harmful effects are genetic mutation, anemia, reproductive disorders (fetal death or abnormality), cataracts, and skin irritation. It can cause respiratory tract irritation if inhaled, skin irritation if it contacts the skin and eye irritation if it contacts the eyes. As a reproductive toxin BrDU would be considered a “particularly hazardous substance” under the OSHA lab standard. Interactions 5-Bromo-2'-deoxyuridine (BrdUrd) was found to increase the cytotoxicity induced by 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and cisplatin in human glioma cells. At a fixed concentration of BrdUrd and BCNU, the greatest cell loss was observed in exponentially growing cells. As cells approached plateau growth, cytotoxicity was reduced as indicated by greater cell viability. Under varying growth conditions the percentage of thymine replacement by bromouracil in DNA, as determined by gas chromatography/mass spectrometry analysis, declined as cultures approached maximum density. These data indicate BrdUrd must be incorporated into DNA for the enhanced effect to be observed. In exponentially growing cells, sensitization was dependent upon both the concentration of BrdUrd and alkylating agent. Using regression analysis (at 95% CL), a relationship between the level of bromouracil in DNA and the extent of enhanced cytotoxicity was observed at two concentrations of BCNU (r2 = 0.99, 0.96). Although it is known that bifunctional alkylating agents exert cytotoxicity by forming cross-links between cDNA strands, increased cross-link formation was not observed in BrdUrd substituted DNA as determined by alkaline elution. The data suggest that DNA damage induced by halogenated pyrimidines may not involve interstrand cross-links and that these agents may be useful in the treatment of glioma in combination with alkylating agents. ToxicityData Rat(po): LD50: 8400 mg/kg Rat(ip): LD50: 1500 mg/kg Toxicity Data Rat(po): LD50: 8400 mg/kg Rat(ip): LD50: 1500 mg/kg Rat(sc): LD50: 3900 mg/kg Rat(iv): LD50: 2320 mg/kg Mouse(po): LD50: 9100 mg/kg Mouse(ip): LD50: 3050 mg/kg Mouse(sc): LD50: 3500 mg/kg Mouse(iv): LD50: 2500 mg/kg LD50: 2500 mg/kg (Intravenous, Mouse) (T14) LD50: 3500 mg/kg (Subcutaneous, Mouse) (T14) LD50: 3050 mg/kg (Intraperitoneal, Mouse) (T14) LD50: 9100 mg/kg (Oral, Mouse) (T14) Interactions 5-Bromo-2'-deoxyuridine (BrdUrd) was found to increase the cytotoxicity induced by 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and cisplatin in human glioma cells. At a fixed concentration of BrdUrd and BCNU, the greatest cell loss was observed in exponentially growing cells. As cells approached plateau growth, cytotoxicity was reduced as indicated by greater cell viability. Under varying growth conditions the percentage of thymine replacement by bromouracil in DNA, as determined by gas chromatography/mass spectrometry analysis, declined as cultures approached maximum density. These data indicate BrdUrd must be incorporated into DNA for the enhanced effect to be observed. In exponentially growing cells, sensitization was dependent upon both the concentration of BrdUrd and alkylating agent. Using regression analysis (at 95% CL), a relationship between the level of bromouracil in DNA and the extent of enhanced cytotoxicity was observed at two concentrations of BCNU (r2 = 0.99, 0.96). Although it is known that bifunctional alkylating agents exert cytotoxicity by forming cross-links between cDNA strands, increased cross-link formation was not observed in BrdUrd substituted DNA as determined by alkaline elution. The data suggest that DNA damage induced by halogenated pyrimidines may not involve interstrand cross-links and that these agents may be useful in the treatment of glioma in combination with alkylating agents. Non-Human Toxicity Values LD50 Mouse iv 2500 mg/kg LD50 Mouse sc 3500 mg/kg LD50 Mouse ip 3050 mg/kg LD50 Mouse oral 9100 mg/kg For more Non-Human Toxicity Values (Complete) data for BROMODEOXYURIDINE (8 total), please visit the HSDB record page. |
||
| 参考文献 | |||
| 其他信息 |
Therapeutic Uses
Orphan Drug. Drug Trade name Broxine/Neomark. Used for radiation sensitivity in the treatment of primary brain tumors. The halogenated pyrimidine analogs, bromodeoxyuridine (BUdR) and iododeoxyuridine (IUdR) have been recognized as potential clinical radiosensitizers for over two decades. In vivo and in vitro experimental studies document that radiosensitization is directly dependent on the amount of thymidine replacement in DNA by these analogs. ... Carcinogenicity has not been demonstrated; in fact, it is a useful agent in the treatment of neoplasms because it sensitizes tumor cells to the lethal effects of X-rays to a greater degree than normal tissue cells. Antineoplastic adjunct (radiosensitizer); diagnostic aid (tumor cell label for cytokinetic analysis). For more Therapeutic Uses (Complete) data for BROMODEOXYURIDINE (9 total), please visit the HSDB record page. Drug Warnings /The authors/ report here the results of a Phase I study conducted to determine the toxicity and serum levels that could be tolerated by patients receiving i.v. bromodeoxyuridine concomitantly with radiation therapy. Because of severe thrombocytopenia and leukopenia that was produced in three patients treated by a 96 hour infusion of bromodeoxyuridine at a dose of 1.5 g/sq m/24 hours, the dose was reduced to 0.8 g/sq m/24 hours in these patients and the remaining 9 patients in the study group. Even at this dosage, myelotoxicity was observed. During a clinical Phase I study of bromodeoxyuridine (BUdR) as a radiation sensitizer ... the normal and malignant cells that incorporated the BUdR /were identified/. BUdR was infused for up to 14 days and the in vivo incorporation of BUdR into DNA was assessed using an immunohistochemical technique and a monoclonal antibody directed against BUdR. BUdR was identified in 50% of breast cancer cells and 10% of cells in a malignant melanoma. BUdR was also found in the basal layer of the normal epidermis and in 50% of cells in the marrow. The incorporation of BUdR into cells in the epidermis and marrow may produce the phototoxicity and myelosuppression observed in patients treated with BUdR. ... Twelve patients were treated with continuous intravenous (24-hour) infusions of bromodeoxyuridine (BUdR) at 650 or 1,000 mg/sq m/d for up to two weeks. Myelosuppression, especially thrombocytopenia, was the major systemic toxicity and limited the infusion period to nine to 14 days. However, bone marrow recovery occurred within seven to ten days, allowing for a second infusion in most patients. Local toxicity (within the radiation field) was minimal, with the exception of one of four patients, who underwent abdominal irradiation. Pharmacology studies revealed a steady-state arterial plasma level of 6 X 10-7 mol/L and 1 X 10-6 mol/L during infusion of 650 and 1,000 mg/sq m/d, respectively. In vivo BUdR uptake into normal bone marrow was evaluated in two patients by comparison of preinfusion and postinfusion in vitro radiation survival curves of marrow CFUc with enhancement ratios (D0-pre/D0-post) of 1.8 (with 650 mg/sq m/d) and 2.5 (with 1,000 mg/sq m/d). In vivo BUdR incorporation into normal skin and tumor cells using an anti-BUdR monoclonal antibody and immunohistochemistry was demonstrated in biopsies from three patients revealing substantially less cellular incorporation into normal skin (less than 10%) compared with tumor (up to 50% to 70%). We conclude that local and systemic toxicity of continuous infusion of BUdR at 1,000 mg/sq m/d for approximately two weeks is tolerable. The observed normal tissue toxicity is comparable with our previous clinical experience with intermittent (12 hours every day for two weeks) infusions of BUdR. Theoretically, a constant infusion should allow for greater incorporation of BUdR into cycling tumor cells and thus, for further enhancement of radiosensitization. ... 12 hours of BUdR at a dose of 800-1,000 mg/sq m for five days a week was given to 23 patients with primary and secondary malignant brain tumors during radiation therapy. Radiation therapy was planned at a weekly dose of 10 Gy for five to six weeks. Fifteen patients received 1,000 mg/sq m of BUdR; six of them tolerated more than three weeks of treatment. In eight patients given doses of 800 mg/sq m, five patients tolerated more than three weeks. The most remarkable toxic effects were myelosuppression and stomatitis, which were major obstacles to maintaining the schedule. It is cytotoxic, strongly teratogenic, and mutagenic in some test systems. |
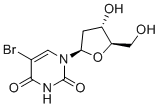
| 分子式 |
C9H11BRN2O5
|
|
|---|---|---|
| 分子量 |
307.1
|
|
| 精确质量 |
305.985
|
|
| 元素分析 |
C, 35.20; H, 3.61; Br, 26.02; N, 9.12; O, 26.05
|
|
| CAS号 |
59-14-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
6035
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.9±0.1 g/cm3
|
|
| 熔点 |
191-194 °C (dec.)(lit.)
|
|
| 折射率 |
1.652
|
|
| LogP |
-0.81
|
|
| tPSA |
104.55
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
386
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
BrC1C(N([H])C(N(C=1[H])[C@@]1([H])C([H])([H])[C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])=O)=O
|
|
| InChi Key |
WOVKYSAHUYNSMH-RRKCRQDMSA-N
|
|
| InChi Code |
InChI=1S/C9H11BrN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
|
|
| 化学名 |
5-bromo-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 14.29 mg/mL (46.53 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2563 mL | 16.2813 mL | 32.5627 mL | |
| 5 mM | 0.6513 mL | 3.2563 mL | 6.5125 mL | |
| 10 mM | 0.3256 mL | 1.6281 mL | 3.2563 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00001650 | Completed | N/A | Acquired Immunodeficiency Syndrome HIV Infection |
National Institute of Allergy and Infectious Diseases (NIAID) |
May 13, 2011 | N/A |
| NCT00003832 | Completed | Procedure: conventional surgery Drug: bromodeoxyuridine |
Stage I Prostate Cancer Stage IIA Prostate Cancer |
National Cancer Institute (NCI) |
July 1999 | Phase 2 |
|
|
|
|