| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |

| 体内研究 (In Vivo) |
丁基羟基甲苯(BHT)有效促进肿瘤诱发的肿瘤这一事实已得到广泛认可。在 7 周大的肿瘤中,丁基羟基甲苯(面部;400 毫克/公斤;每周)制剂可增强 rasH2 肿瘤发生的功效。感性 [3]
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The biliary metabolism of BHT, BHT-COOH, BHT-OH, and BHT-aldehyde was compared after i.p. or intravenous administration to male Wistar rats. For all four test compounds, the major metabolites in enterohepatic circulation were BHT-COOH and its ester glucuronide. ... Total biliary excretion after treatment with BHT or BHT-aldehyde was less after i.v. dosing than after i.p. dosing. ... Rats and humans received single doses of BHT. Male Wistar rats (n=2-10) were treated with 20 to 200 mg/kg BHT. Human subjects were treated with 0.5 mg/kg BHT (seven non-smoking males). In rats, kinetic parameters increased dose-dependently. Rats excreted ~10% of the high dose as unchanged BHT in the feces, mostly on day 1. Urinary excretion of BHT-COOH was little more than 1%, in decreasing amounts, on days 1 to 4. In humans, the mean plasma concentration-time profile was decreased as compared to that of rats. Unchanged BHT was not detected in the feces, and urinary excretion of BHT-COOH was 0 to 5.5%. After a single dose of [(14)C]BHT /to human males/ at least two-thirds of the radioactivity is excreted in urine, with the major portion appearing within 24 hr of dosing. As occasional assays demonstrated a fecal excretion of about one-half of that in urine, it is probable that the remainder of the radioactivity was excreted by this route. The bulk of the radioactivity appears on the first day after dosing, and thereafter a progressively diminishing slight excretion continues for a considerable period. ... Solutions of BHT (10 mg/L) were prepared in polyethylene glycol 400-normal saline (1:1). The solutions were infused in rabbits for <2 minutes at a constant rate of 2 mL/min to provide a total dose of 10 mg/kg. Blood samples were collected at 0, 0.085, 0.173, 0.33, 0.50, 0.75, 1, 1.15, 2.0, 3.0, 4.0, 6.0 and 12 hours and thereafter twice per day for 2 days and daily for 3 days. Blood samples were analyzed for their concentration of BHT using a highly sensitive and specific GLC method. The fast disposition phase half-life of BHT was approximately 1 hour and the slow disposition phase decayed with a half-life of >11 days. These data suggest rapid accumulation and slow clearance from the body. BHT tends to be stored in body tissues upon multiple dosing and more than a 16-fold accumulation of BHT is possible on daily exposure. For more Absorption, Distribution and Excretion (Complete) data for 2,6-DI-T-BUTYL-P-CRESOL (7 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of BHT has been investigated extensively in rabbits, rat, mice and man. The principle routes of metabolism of BHT in all species involve oxidation of the para-methyl and of one, or both, of the tert-butyl substituents. Neither mechanism is mutually exclusive. Oxidation of the methyl-group is catalyzed by the microsomal enzyme, BHT-oxidase and several derivatives including the quinone-methide, 2,6-di-tert-butyl-4-methylene-2,5-cyclohexadienone and 4-hydroxy-4-methyl-2,6-di-tert-butyl-cyclahexe-2,5-dienone have been identified in rat liver. Whereas oxidation of the para-methyl substituent is the major route of metabolism in the rat and rabbit, where BHT-acid accounts for approximately 30% of the dose, some 30-40% of the dose in male and female mice and in man is excreted as metabolites involving oxidation of one or both of the tert-butyl groups. BHT is excreted principally in the urine in man whereas in rodents 50-80% is eliminated in the feces. This is presumed to be due to species differences in the molecular weight threshold for biliary excretion. The biliary metabolism of BHT, BHT-COOH, BHT-OH, and BHT-aldehyde was compared after i.p. or intravenous administration to male Wistar rats. For all four test compounds, the major metabolites in enterohepatic circulation were BHT-COOH and its ester glucuronide. ... Total biliary excretion after treatment with BHT or BHT-aldehyde was less after i.v. dosing than after i.p. dosing. ... A comparative metabolism study of BHT was conducted in mice and rats. In male and female DDY/Slc mice given single oral doses (20 or 500 mg/kg body weight) of BHT labelled with (14)C at the p-methyl group, (14)C was distributed mainly in the stomach, intestines, liver and kidney, and then excreted in the urine, feces and expired air. During the 7 days after treatment, 41-65, 26-50 and 69% of the (14)C dose was excreted in feces, urine and expired air, respectively, and the total recovery was 96-98%. Levels of (14)C in 21 male and 22 female tissues 7 days after treatment were less than 1 ug BHT equivalents/g tissue (ppm) in mice given 20 mg/kg and less than 11 ppm in mice given 500 mg/kg. When [(14)C]BHT was given orally to male mice at 20 mg/kg/day for 10 days, (14)C was rapidly excreted and did not exhibit any tendency to accumulate in any tissues. Thin-layer chromatography and high-performance liquid chromatography analyses showed that more than 43 metabolites were present in the urine and feces of both species, and all of these were identified to determine metabolic pathways for BHT in mice and rats. Major metabolic reactions of [(14)C]BHT in mice were the oxidation of the p-methyl group attached to the benzene nng and of the tert-butyl groups. The products from the latter reaction were cyclized to some extent by reacting with the adjacent phenolic OH group to give hemiacetals or lactones. The carboxyl derivatives from the p-methyl oxidation were conjugated with glucuronic acid. When single oral doses of 20 or 500 mg [(14)C]BHT/kg were given to male Sprague-Dawley rats, metabolites similar to those in mice were found. However, the major biotransformation was oxidation of the p-methyl group, and oxidation of the tert-butyl groups was a minor reaction in rats. The principal metabolites of 2,6-di-tert-butyl-p-cresol (BC) in mouse bronchiolar Clara cells were 6-tert-butyl-2-(hydroxy-tert-butyl)p-cresol (BC-butOH; 4.4 +/- 1.1 pmol/10-6 cells/minute) and 2,6-di-tert-butyl-p-hydroxymethyl-phenol (BC-OH; 1.0 +/- 0.2 pmol/10-6 cells/minute). This metabolite pattern is nearly identical with that obtained with microsomes prepared from whole lungs. Quinone methide production occurred more readily from BC-butOH than from BC (0.52 +/- 0.14 compared to 0.41 +/- 0.06 pmol/10-6 cells/minute). The maximum concentration of the intermediate BC-butOH was very low relative to that of BC; similar quantities of the quinone methides were generated. Furthermore, two glutathion conjugates, expected from attack of BC-quinone methide and BC-butOH-quinone methide, were found. Incubation time was 15 minutes (Clara cells) or 10 minutes (microsomes). Metabolite of BHT isolated from liver of orally treated male rats. Identified as 2,6-di-tert-butyl-4-methylene-2,5-cyclohexadienone. Oxidative metabolism (phase 1 reactions) mediated by the microsomal monooxygenase system is the major route for BHT degradation. Oxidation of the tert-butyl groups is most common in man. Gallates and 2-tert-butylhydroquinone are mainly metabolized by non-oxidative pathways (methylation or conjugation with sulphate and glucuronic acid). (A15352). In particular BHT is frequently metabolized to quinone methides (QMs) which are thought to be responsible for promoting tumor formation. One example of a QM is 2,6-di-tert-butyl-4-methylenecyclohexa-2,5-dienone (BHT-QM). QMs are strongly electrophilic and readily form adducts with proteins. Biological Half-Life Solutions of BHT (10 mg/L) were prepared in polyethylene glycol 400-normal saline (1:1). The solutions were infused in rabbits for <2 minutes at a constant rate of 2 mL/min to provide a total dose of 10 mg/kg. ... The fast disposition phase half-life of BHT was approximately 1 hour and the slow disposition phase decayed with a half-life of >11 days. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Butylated hydroxytoluene( BHT) is a white, crystalline, odorless solid. It is used as an antioxidant for fats and oils or in packaging material for fat containing foods. HUMAN EXPOSURE AND TOXICITY: Potential symptoms of overexposure are irritation of eyes and skin. ANIMAL STUDIES: Rats fed high doses of BHT, showed increases in serum cholesterol in both sexes. Groups of weanling rats fed BHT in conjunction with lard supplementation had a reduction in growth rate, especially in males. BHT also increased absolute liver weight and the ratio of liver weight to body weight in both sexes. BHT increased the ratio of left adrenal weight to body weight in male rats but had no consistent effect in female rats. BHT administered to rats for 68-82 days caused reduction in rate of increase in weight and fatty infiltration of the liver. BHT was given in feed of rats and mice of both sex at 3000 or 6000 ppm; in rats 105 wk and 107 or 108 wk in mice. No tumors occurred in either sex of rats and mice. When tested for teratogenic properties BHT produced anophthalmia in offspring in rats, but not in mice. BHT administered to pregnant mice for 18 days along with another group fed BHT for 50 to 64 days including 18 das of pregnancy. No fetal abnormalities were observed. In a study using 144 mice, no blindness was observed in any of the 1162 litters representing 7765 offspring born throughout the reproductive life span of the mothers. BHT was tested for mutagenicity in the Salmonella/microsome preincubation assay in 5 Salmonella typhimurium strains (TA1535, TA1537, TA97, TA98, and TA100) in the presence and absence of metabolic activation. BHT was negative in these tests and the highest ineffective dose tested in any Salmonella typhimurium strain was 10 mg/plate. ECOTOXICITY STUDIES: In salmon fed graded levels of BHT during a 12-week feeding followed by a 2-week depuration period, BHT selectively modulated toxicological responses in the xenobiotic biotransformation pathways during the feeding period. BHT is metabolized to quinone methides (QMs) which are responsible for promoting tumor formation in many animal models. One example of a QM is 2,6-di-tert-butyl-4-methylenecyclohexa-2,5-dienone (BHT-QM). QMs are strongly electrophilic and readily form adducts with proteins. Some of the QM targets include redox proteins such as glutathione S-transferase P1 (GST-P1), peroxiredoxin 6 (Prx6), Cu,Zn-superoxide dismutase (SOD1), carbonyl reductase, and selenium-binding protein 1, which have direct or indirect antioxidant functions. (A15087, A15355). The modification of these proteins leads to decreased cellular protection from electrophiles and oxidants. Alkylation also may interfere with GSTP1 regulation of stress kinases, thereby influencing phosphorylation and cell growth. BHT also binds to the retinoic acid receptor which can lead to changes in cell development. Interactions During the DPPH scavenging assay carried out in non polar and non protic solvents, such as toluene, BHT regenerates alpha-tocopherol from tocopheryl radical, whereas in polar and protic solvents, like methanol, no regeneration is observed due to a fast electron transfer reaction from the tocopheryl radical to the reactive DPPH radical. ... In the presence of a small amount of alcohol, the synergy is exalted and BHT regenerates twice as much alpha-tocopherol due to a nucleophilic addition of short alcohols on the BHT oxidation product, giving a new phenolic co-antioxidant. The natural retinoid, retinyl acetate (RA), and the phenolic antioxidant, butylated hydroxytoluene (BHT), are both effective inhibitors of mammary carcinogenesis in rats. The present study was designed to determine if an increased inhibition of mammary carcinogenesis is obtained when RA and BHT are administered in combination. At age 50 days (time 0), virgin, female Sprague-Dawley rats received a single intragastric instillation of 16 mg of 7,12-dimethylbenz(a)anthracene dissolved in 1 mL sesame oil. Groups of 30 carcinogen-treated rats received Wayne Lab Chow supplemented with (per kg diet) 250 mg RA, 5000 mg BHT, or 250 mg RA plus 5000 mg BHT by the following schedule: -2 to +1 week; +1 week until the end of the experiment; -2 weeks to end; or none. Combined administration of RA plus BHT by the -2 weeks to end schedule was more effective in mammary cancer chemoprevention than was RA alone or BHT alone; the interaction of RA and BHT was additive. Similarly, administration of RA plus BHT by the -2 weeks to end protocol was more active in chemoprevention than was RA plus BHT administered either from weeks -2 to +1 or +1 week to end. Chronic exposure to RA plus BHT induced a high incidence of hepatic fibrosis and bile duct hyperplasia; these changes were not observed in controls and were seen in low incidence in animals exposed to RA only or BHT only. These data indicate that enhanced anticarcinogenic activity can be obtained through the use of "combination chemoprevention" regimens; however, chemopreventive compounds may interact not only to inhibit carcinogenesis but also to induce toxicity. The individual and combined (binary mixtures) (anti)androgenic effect of butylparaben (BuPB), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate (PG) was evaluated using the MDA-kb2 cell line. Exposing these cells to AR agonists results in the expression of the reporter gene (encoding for luciferase) and luminescence can be measured in order to monitor the activity of the reporter protein. In case of the evaluation of the anti-androgenic effect, the individual test compounds or binary mixtures were tested in the presence of a fixed concentration of a strong AR agonist (1000 pM 5-alpha-dihydrotestosterone; DHT). Cell viability was assessed using a resazurin based assay. For PG, this is the first report in the literature concerning its (anti)androgenic activity. In case of both individual and mixture testing none of the compounds or binary combinations showed androgenic activity. When tested in the presence of DHT, BuPB, BHA and BHT proved to be weak anti-androgens and this was confirmed during the evaluation of binary mixtures (BuPB+BHA, BuPB+BHT and BHA+BHT). Besides performing the in vitro testing of the binary combinations, two mathematical models (dose addition and response addition) were evaluated in terms of accuracy of prediction of the anti-androgenic effect of the selected binary mixtures. The dose addition model guaranteed a good correlation between the experimental and predicted data. However, no estimation was possible in case of mixtures containing PG, due to the lack of effect of the compound in case of the individual testing. The aim of this study was to compare the effects of L-arginine (L-arg) and food-antioxidant butylated hydroxytoluene (BHT) against oxidative stress of Escherichia coli endotoxin (LPS) in the liver. Ninety Wistar albino rats were assigned in three groups. Rats received one of the following pre-treatment previous to 5 mg/kg LPS intraperitoneally: saline, L-arg (NO donor, 100 mg/kg), or BHT (250 mg/kg/day), for 3 days. At second, fourth and sixth hours, plasma nitrite-plus-nitrate, circulating liver enzymes, glutathione levels, superoxide dismutase, glutathione peroxidase activities were measured. The most remarkable liver injury was evident in BHT pre-treated animals at all time points compared to L-arg pre-treated rats. While BHT enhanced superoxide dismutase activities following LPS, glutathione decreased simultaneously compared to L-arg group. ... For more Interactions (Complete) data for 2,6-DI-T-BUTYL-P-CRESOL (15 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 890 mg/kg LD50 Mouse oral 650 mg/kg LD50 Mouse ip 138 mg/kg LD50 Mouse iv 180 mg/kg For more Non-Human Toxicity Values (Complete) data for 2,6-DI-T-BUTYL-P-CRESOL (11 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
/EXPL THER/ /The objective was to/ evaluate the efficacy of potential therapeutics in Rdh8(-/-)Abca4(-/-) mice, a rodent model of human age-related macular degeneration (AMD). Therapeutic efficacy of several antioxidant agents (ascorbic acid, alpha-lipoic acid, alpha-tocopherol, Mn(III)-tetrakis(4-benzoic acid)-porphyrin, and butylated hydroxytoluene), an immunosuppressive agent with antivascular endothelial growth factor (VEGF) activity (sirolimus, also known as rapamycin), a retinoid cycle inhibitor (retinylamine), and an artificial chromophore (9-cis-retinyl acetate) were evaluated side by side in a recently described murine model of AMD, the Rdh8(-/-)Abca4(-/-) mouse. This animal exhibits a retinopathy caused by delayed all-trans-retinal clearance resulting from the absence of both ATP-binding cassette transporter 4 (Abca4) and retinol dehydrogenase 8 (Rdh8) activities. Drug efficacy was evaluated by retinal histologic analyses and electroretinograms (ERGs). All tested agents partially prevented atrophic changes in the Rdh8(-/-)Abca4(-/-) retina with retinylamine demonstrating the greatest efficacy. A significant reduction of complement deposition on Bruch's membrane was observed in sirolimus-treated mice, although the severity of retinal degeneration was similar to that observed in antioxidant- and 9-cis-retinyl acetate-treated mice. Sirolimus treatment of 6-month-old Rdh8(-/-)Abca4(-/-) mice for 4 months prevented choroidal neovascularization without changing retinal VEGF levels. Mechanism-based therapy with retinylamine markedly attenuated degenerative retinopathy in Rdh8(-/-)Abca4(-/-) mice. ... /EXPL THER/ The present study was undertaken to evaluate the possible ameliorating effect of butylated hydroxyl toluene (BHT), associated with ferric nitrilotriacetate (Fe-NTA)-induced oxidative stress and liver injury in mice. The treatment of mice with Fe-NTA alone enhances ornithine decarboxylase activity to 4.6 folds, protein carbonyl formation increased up to 2.9 folds and DNA synthesis expressed in terms of [(3)H] thymidine incorporation increased to 3.2 folds, and antioxidants and antioxidant enzymes decreased to 1.8-2.5 folds, compared with the corresponding saline-treated controls. These changes were reversed significantly (p < 0.001) in animals receiving a pretreatment of BHT. Our data show that BHT can reciprocate the toxic effects of Fe-NTA and can serve as a potent chemopreventive agent. |
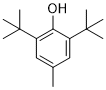
| 分子式 |
C15H24O
|
|---|---|
| 分子量 |
220.36
|
| 精确质量 |
220.182
|
| CAS号 |
128-37-0
|
| 相关CAS号 |
Butylated hydroxytoluene-d21;64502-99-4;Butylated hydroxytoluene-d24;1219805-92-1;Butylated hydroxytoluene-d3;86819-59-2
|
| PubChem CID |
31404
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.048
|
| 沸点 |
265 ºC
|
| 熔点 |
69-71 ºC
|
| 闪点 |
127 ºC
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.499
|
| LogP |
5.32
|
| tPSA |
20.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
207
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
NLZUEZXRPGMBCV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3
|
| 化学名 |
Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl-
|
| 别名 |
Butylated hydroxytoluene NSC-6347 NSC6347NSC 6347
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~453.82 mM)
H2O : ~1 mg/mL (~4.54 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (11.35 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (11.35 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (11.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (453.82 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5380 mL | 22.6901 mL | 45.3803 mL | |
| 5 mM | 0.9076 mL | 4.5380 mL | 9.0761 mL | |
| 10 mM | 0.4538 mL | 2.2690 mL | 4.5380 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02221375 | COMPLETED | Drug: BHT low Drug: BHT medium Drug: BHT high |
Healthy | Boehringer Ingelheim | 2008-06 | Phase 1 |
| COMPLETED | COMPLETED | Drug: BHT 0.1% Drug: BHT 0.5% Drug: Placebo for RMT-B Drug: Placebo for HFA-MDI |
Asthma | Boehringer Ingelheim | 2009-11 | Phase 1 |
| NCT03547206 | TERMINATED | Drug: RPh201 Cohort A Other: Placebo Cohort A Drug: RPh201 Cohort B Other: Placebo Cohort B |
Nonarteritic Anterior Ischemic Optic Neuropathy | Regenera Pharma Ltd | 2018-07-10 | Phase 2 |
| NCT02578953 | COMPLETED | Drug: Dutasteride-Test product Drug: Dutasteride-Reference product |
Prostatic Hyperplasia | GlaxoSmithKline | 2015-09-09 | Phase 1 |
| NCT03105505 | UNKNOWN STATUS | Drug: Permethrin 5% Drug: Synthomycine 5% Drug: Fusidic Acid 1% M/R Eye Drops |
Inflammation of the Eyelids | Barzilai Medical Center | 2017-04-28 | Phase 4 |