| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
DNA Alkylator
|
|---|---|
| 体外研究 (In Vitro) |
卡莫司汀是一种用于治疗癌症的化疗药物。卡莫司汀(8、80 和 800 μM)均可降低神经元细胞增殖、肿瘤细胞质以及 2-氨基苯甲酸 (AF) 和对氨基苯甲酸 (PABA) 的完整 N-苯甲酰转移酶 (NAT) 活性。 DNA-AF 加成复合物随着肿瘤神经生长细胞的发育而升高,而卡莫司汀则降低它[1]。
卡莫司汀和洛莫司汀是亚硝基脲类抗肿瘤化疗药物,研究了它们对大鼠神经胶质瘤细胞系(C6胶质瘤)中芳胺n -乙酰基转移酶(NAT)活性和dna -2-氨基芴加合物的影响。采用高效液相色谱法测定n -乙酰基-2-氨基芴(AAF)和n -乙酰基-对氨基苯甲酸(N-Ac-PABA)的残留量以及2-氨基芴(AF)和对氨基苯甲酸(PABA)的残留量。结果表明,卡莫司汀和洛莫司汀使胶质肿瘤细胞胞浆和完整肿瘤细胞的NAT活性呈剂量依赖性降低。卡莫司汀和洛莫司汀联合处理后,大鼠胶质肿瘤细胞内NAT的表观值Km和Vmax均降低。将神经胶质肿瘤细胞暴露于不同浓度的AF(与卡莫司汀和洛莫司汀联合或不联合)后,采用gamma-[32p]- datp和HPLC测定DNA-AF加合物。卡莫司汀和洛莫司汀共同作用可降低大鼠神经胶质肿瘤细胞中DNA-AF加合物的表达。本报告首次证实卡莫司汀和洛莫司汀抑制大鼠胶质肿瘤细胞NAT活性和DNA-AF加合物形成[1]。 |
| 体内研究 (In Vivo) |
与支架水平 (GSSG) 和还原型谷胱甘肽 (GSH)/GSSG 值相比,卡莫司汀 (BCNU;25 mg/kg,腹膜内注射) 导致死亡与体重、结合胆红素比率、外胆汁流量和氧化型谷胱甘肽[2]。
本研究探讨了抗氧化剂曲美他嗪(TMZ)对卡莫司定(BCNU)所致大鼠肝内胆汁淤积的影响。将大鼠分为四组。第一组(生理盐水)12只大鼠,在实验前48 h腹腔注射生理盐水2 ml/kg。第二组(玉米油组,n=15),在研究前48 h注射2 ml/kg的玉米油IP。第三组(BCNU组,n=16),研究前48 h注射玉米油2 ml/kg + BCNU IP 25 mg/kg。第四组(TMZ组,n=12),每天注射TMZ IP 2.5 mg/kg,每天同一小时单剂量给药。第一次给药TMZ 12 h后,注射玉米油2 ml/kg+BCNU 25 mg/kg IP,玉米油+BCNU给药48 h后纳入研究。戊巴比妥麻醉后,切开腹部,置管于胆总管通道内,每小时测量胆汁量。然后取心内血,离心得到血浆。最后,颈椎脱位处死大鼠,取肝称重。除了肝脏的组织病理学检查外,还检测了丙二醛(MDA)、氧化谷氨酸(GSSG)和减少谷氨酸(GSH)的水平。同时以mOsm/kg计算胆汁和血浆渗透压。结果BCNU组胆流量减少(P<0.005), TMZ组胆流量正常。BCNU组大鼠血清结合胆红素水平高于其他各组(P<0.05)。虽然TMZ组总谷氨酸水平较低(P<0.005),但GSH/GSSG比值正常。这些发现表明TMZ对BCNU引起的肝内胆汁淤积有保护作用。[2] |
| 酶活实验 |
2-氨基芴 (AF) 和对氨基苯甲酸 (PABA) N-乙酰化以乙酰辅酶 A 依赖性方式测定。测定系统的孵育混合物总体积为 90 μL,包括神经胶质肿瘤细胞胞质溶胶,根据需要稀释在 50 μL 裂解缓冲液(20 mM Tris/HCl,pH 7.5,1 mM DTT 和 1 mM EDTA)中,20 μL指定浓度的 50 mM Tris-HCl (pH7.5)、0.2 mM EDTA、2 mM DTT、15 mM 乙酰肉碱、2U/mL 肉毒碱乙酰转移酶和 AF 或 PABA 的乙酰辅酶A回收混合物。添加 20 μL 乙酰辅酶A 启动反应。对照反应中的乙酰辅酶A被 20 μL 蒸馏水替代。用于单点活性测量的 AcCoA 和 PABA 的最终浓度分别为 0.5 mM 和 0.1 mM。将反应混合物(含或不含特定浓度的卡莫司汀和洛莫司汀)在 37° 下孵育 10 分钟后,使用 50 μL 20% 三氯乙酸终止 PABA 反应,并使用 100 μL 乙腈终止 AF 反应。 C。每个反应,包括对照和实验,均进行三次[1]。
|
| 动物实验 |
Rats: Rats are randomly assigned to four groups after being weighted individually before beginning the study and having their weights recorded. There are twelve rats in Group I (the saline group). The study includes the rats 48 hours after they receive an intraperitoneal (IP) injection of 2 mL/kg of saline 48 hours prior to the study. Fifteen rats make up Group II (corn oil group). The rats receive a 2 mL/kg injection of corn oil (vehicle) IP 48 hours prior to the investigation. Sixteen rats make up Group III (Carmustine group). For three days, the same hour of the day, a single-dose of 1 mL of saline IP is injected into these rats. The rats are added to the study 48 hours after the first dose of saline is administered, and twelve hours later, they receive injections of corn oil (2 mL/kg) and carmustine (25 mg/kg IP). There are twelve rats in Group IV (the trimetazidine group). For three days, these rats receive a single-dose injection of 2.5 mg/kg of trimetazidine (TMZ) IP at the same hour every day. Corn oil (2 mL/kg) and carmustine (25 mg/kg IP) are injected 12 hours after the first dose of TMZ, and the rats are added to the study 48 hours later[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
5 to 28% bioavailability Approximately 60% to 70% of a total dose is excreted in the urine in 96 hours and about 10% as respiratory CO2. Following IV infusion of carmustine, the steady-state volume of distribution averaged 3.25 L/kg. Because of their high lipid solubility, carmustine and/or its metabolites readily cross the blood-brain barrier. Substantial CSF concentrations occur almost immediately after IV administration of carmustine, and CSF concentrations of radioactivity have been variously reported to range from 15-70% of concurrent plasma concentrations. Carmustine metabolites are distributed into milk, but in concentrations less than those in maternal plasma. The absorption of the copolymer contained in carmustine wafers has not been evaluated in humans. Plasma concentrations of carmustine following intracranial implantation of the wafers have not been determined in humans, but in rabbits undergoing surgical implantation of wafers containing 3.85% carmustine, no detectable levels of carmustine were observed in plasma. When the carmustine wafer is exposed to the aqueous environment of the resection cavity, hydrolysis of the anhydride bonds in the copolymer occurs, resulting in the release of carmustine and two monomers, carboxyphenoxypropane, and sebacic acid. The carmustine contained in the wafer diffuses into the surrounding brain tissue. The metabolism and excretion of the copolymer contained in carmustine wafers has not been evaluated in humans. Animal studies have shown that more than 70% of the copolymer degrades within 3 weeks following implantation of carmustine wafers into brain tissue; following hydrolysis of the copolymer, carboxyphenoxypropane is eliminated renally, while sebacic acid (an endogenous fatty acid) is metabolized in the liver and expired as carbon dioxide. In humans, wafer remnants have been observed on brain imaging scans or located during subsequent surgical procedures up to 8 months following intracranial implantation. Wafer remnants retrieved from 2 patients approximately 2-3 months after implantation were analyzed and found to consist mostly of water and monomeric components with minimal detectable amounts of carmustine. The disappearance of 1,3-bis(2-chlorethyl)-1-nitrosourea (BCNU) from plasma, liver, kidney, lung, brain, spleen, tumor tissue and epididymal adipose tissue of Walker 256/B carcinoma-bearing rats and healthy animals was measured by differential pulse polarography after i.v. bolus of the drug. Only BCNU, not its decomposition products, was detected by the polarographic assay. Levels of BCNU in liver of tumor-bearing animals were significantly lower (about 10 times) than those on healthy rats. A bi-exponential fit was used to calculate the kinetics of BCNU in plasma, kidney, lung and brain, but no difference could be found between healthy and Walker tumor-bearing rats. BCNU disappeared faster from adipose tissue of tumor-bearing animals than from normals. Some 40 minutes after injection, BCNU is no longer an effective antitumour agent, and a few minutes after administration no unchanged BCNU can be detected in plasma. Following its ip or sc injection or oral administration, BCNU was rapidly distributed to most tissues, including brain and cerebrospinal fluid. Excretion was primarily in the urine; it was most rapid in mice (80% of the dose excreted in 24 hours) and less rapid in monkeys and dogs. Metabolism / Metabolites Hepatic and rapid with active metabolites. Metabolites may persist in the plasma for several days. The in vitro metabolism of the anticancer agent 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) has been studied in male Fischer 344 rat liver microsomal preparations. The previously identified product, 1,3-bis(2-chloroethyl)urea (BCU), has been shown to be the major metabolite. Stable isotope labeling and mass spectral analysis of isolated metabolites indicate that BCU is formed exclusively from the metabolic denitrosation of BCNU. The rate of BCNU chemical decomposition in rat liver microsomal preparations deficient in NADPH and the metabolic disappearance rate in preparations containing added NADPH were measured and compared with the measured rate of metabolic formation of BCU under the same conditions. The rate of NADPH-dependent BCNU metabolism and BCU formation are equal within experimental error. BCNU was found to inhibit the rat liver 9000 g supernatant metabolism of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU). Hepatic and rapid with active metabolites. Metabolites may persist in the plasma for several days. Route of Elimination: Approximately 60% to 70% of a total dose is excreted in the urine in 96 hours and about 10% as respiratory CO2. Half Life: 15-30 minutes Biological Half-Life 15-30 minutes |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Carmustine causes cross-links in DNA and RNA, leading to the inhibition of DNA synthesis, RNA production and RNA translation (protein synthesis). Carmustine also binds to and modifies (carbamoylates) glutathione reductase. This leads to cell death. Hepatotoxicity Mild and transient elevations in serum aminotransferase levels are found in up to 25% of patients treated with carmustine. Because carmustine is typically given in combination with other agents, its role in causing these serum enzyme elevations is not always clear. The abnormalities are generally transient, do not cause symptoms and do not require dose modification. Clinically apparent liver injury from carmustine has been limited to a small number of cases of cholestatic hepatitis and more frequent instances of sinusoidal obstruction syndrome, reported mostly with its use in high doses or as a conditioning agent in preparation for hematopoietic cell transplantation. The onset of sinusoidal obstruction syndrome is usually within two to three weeks of the myeloablation and is characterized by a sudden onset of abdominal pain, weight gain, ascites, marked increase in serum aminotransferase levels (and LDH), and subsequent jaundice and hepatic dysfunction. The severity of sinusoidal obstruction syndrome varies from a transient, self-limited injury to acute liver failure. The diagnosis of sinusoidal obstruction syndrome is usually based on clinical features of tenderness and enlargement of the liver, weight gain, ascites and jaundice occurring within 3 weeks of chemotherapy. Liver biopsy is diagnostic but often contraindicated, because of severe thrombocytopenia after hematopoietic cell transplantation. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury, particularly when used for myeloablation). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of carmustine during breastfeeding. Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, especially alkylating agents such as carmustine. The manufacturer recommends that breastfeeding be discontinued during carmustine therapy and for 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Some evidence indicates that carmustine can increase serum prolactin. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 80% Toxicity Data The oral LD50s in rat and mouse are 20 mg/kg and 45 mg/kg, respectively. Interactions In patients receiving carmustine and phenytoin, serum concentrations of phenytoin may be decreased. In patients receiving carmustine therapy, serum concentrations of phenytoin should be monitored carefully and dosage adjustments made as necessary. Qualitative and quantitative changes in tear films leading to damage of the corneal and conjunctival epithelium have been reported in patients receiving high doses of carmustine and mitomycin. Cimetidine may potentiate the myelosuppressive effects (e.g., neutropenia, agranulocytosis) of myelosuppressive drugs (e.g., alkylating agents, antimetabolites) or therapies (e.g., radiation). Concomitant cimetidine therapy has been reported to potentiate the neutropenic and thrombocytopenic effect of carmustine alone or combined with radiation therapy. Non-Human Toxicity Values LD50 Rat oral 20 mg/kg LD50 Rat ip 17,420 ug/kg LD50 Rat sc 83,200 ug/kg LD50 Rat iv 13,800 ug/kg For more Non-Human Toxicity Values (Complete) data for Carmustine (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
BiCNU is indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following: Brain tumors-glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors. Multiple myeloma-in combination with prednisone. Hodgkin's Disease-as secondary therapy in combination with other approved drugs in patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy. Non-Hodgkin's lymphomas-as secondary therapy in combination with other approved drugs for patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy. bis(Chloroethyl) nitrosourea has been used since 1971 as an antineoplastic agent in the treatment of Hodgkin's lymphoma, multiple myeloma, and primary or metastatic brain tumors. Reported to have antiviral, antibacterial, and antifungal activity, but no evidence was found that it is currently used for these purposes. Former use. MEDICATION (VET): A chemotherapeutic protocol using carmustine in combination with vincristine and prednisone was tested in dogs with multicentric malignant lymphosarcoma. Of seven dogs treated, six (85.7%) achieved complete remission. A partial response occurred in one dog. Median survival time was 224 days (mean 386 days), and median duration of remission was 183 days (mean 323 days). Marked neutropenia was observed following carmustine administration. There were no significant alterations in platelets and red blood cell counts during treatment, and no abnormalities attributable to the chemotherapy were found in serum biochemical profiles. Results of this study showed that carmustine is an effective alternative option in the treatment of canine lymphosarcoma. Drug Warnings /BOXED WARNING/ WARNING: BiCNU (carmustine for injection) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Bone marrow suppression, notably thrombocytopenia and leukopenia, which may contribute to bleeding and overwhelming infections in an already compromised patient, is the most common and severe of the toxic effects of BiCNU. Since the major toxicity is delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose. At the recommended dosage, courses of BiCNU should not be given more frequently than every 6 weeks. The bone marrow toxicity of BiCNU is cumulative and therefore dosage adjustment must be considered on the basis of nadir blood counts from prior dose. Pulmonary toxicity from BiCNU appears to be dose related. Patients receiving greater than 1400 mg/sq m cumulative dose are at significantly higher risk than those receiving less. Delayed pulmonary toxicity can occur years after treatment, and can result in death, particularly in patients treated in childhood. Human systemic effects by parenteral, intravenous, and possibly other routes: nausea or vomiting, reduced white blood cell and blood platelet counts, bone marrow damage, and potentially fatal respiratory system effects, including lung fibrosis, dyspnea, and cyanosis. In a study of 17 children (aged 1-16 years) receiving carmustine in cumulative doses ranging from 770-1800 mg/sq m combined with cranial radiation therapy for intracranial tumors, 8 children (47%) died of delayed pulmonary fibrosis, including all of those who received initial treatment at less than 5 years of age (5 children). Onset of pulmonary fibrosis has been observed up to 17 years following carmustine therapy. Clinical findings include pulmonary hypoplasia with upper zone contraction on chest radiographs, and an unusual pattern of upper zone fibrosis on thoracic CT scans; no abnormal findings were observed on gallium scans.105 Late onset of reduction in pulmonary function was observed in all long-term survivors in the study. Carmustine-induced pulmonary fibrosis may be slowly progressive and cause death. Pulmonary toxicity, including acute or delayed onset of pulmonary fibrosis causing death, has occurred in patients receiving systemic carmustine therapy. Pulmonary toxicity characterized by pulmonary infiltrates and/or fibrosis occurring 9 days to 43 months following treatment has been reported in patients receiving carmustine or related nitrosoureas. Most reported cases of pulmonary toxicity have occurred in patients receiving prolonged carmustine therapy with total doses exceeding 1400 mg/sq m; however, pulmonary fibrosis has occurred with lower total doses. Other risk factors include prior history of pulmonary disease and duration of carmustine therapy. Pulmonary toxicity occasionally has been rapidly progressive and/or fatal. For more Drug Warnings (Complete) data for Carmustine (41 total), please visit the HSDB record page. Pharmacodynamics Carmustine is one of the nitrosoureas indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in treatment of brain tumors, multiple myeloma, Hodgkin's disease, and non-Hodgkin's lymphomas. Although it is generally agreed that carmustine alkylates DNA and RNA, it is not cross resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. |
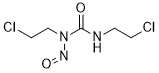
| 分子式 |
C5H9CL2N3O2
|
|---|---|
| 分子量 |
214.0499
|
| 精确质量 |
213.007
|
| 元素分析 |
C, 28.06; H, 4.24; Cl, 33.12; N, 19.63; O, 14.95
|
| CAS号 |
154-93-8
|
| 相关CAS号 |
Carmustine-d8
|
| PubChem CID |
2578
|
| 外观&性状 |
Light yellow solid (low temperature); soild if <30°C; liquid if >30°C
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
404ºC
|
| 熔点 |
30 °C(lit.)
|
| 折射率 |
1.549
|
| LogP |
1.3
|
| tPSA |
61.77
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
156
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC([H])([H])C([H])([H])N(C(N([H])C([H])([H])C([H])([H])Cl)=O)N=O
|
| InChi Key |
DLGOEMSEDOSKAD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11)
|
| 化学名 |
1,3-bis(2-chloroethyl)-1-nitrosourea
|
| 别名 |
NSC409962; NCI-C04773; NCIC04773; NCI C04773; Nitrumon; NSC 409962; NSC-409962; SK 27702; SRI 1720; DTI 015;; FDA 0345; BCNU Becenum; Bi CNU; BiCNU; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Carmubris; Gliadel; Carmustine
|
| HS Tariff Code |
29241900
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 35 mg/mL (~163.5 mM)
H2O: ~100 mg/mL (~467.2 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (9.72 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (9.72 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (9.72 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO+ 40%PEG300+ 5%Tween 80+ 50%ddH2O: 2.0mg/ml (9.34mM) 配方 5 中的溶解度: 100 mg/mL (467.18 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.6718 mL | 23.3590 mL | 46.7181 mL | |
| 5 mM | 0.9344 mL | 4.6718 mL | 9.3436 mL | |
| 10 mM | 0.4672 mL | 2.3359 mL | 4.6718 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Tebentafusp-tebn With LDT in Metastatic UM
CTID: NCT06626516
Phase: Phase 1/Phase 2 Status: Not yet recruiting
Date: 2024-10-15