| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
β-lactam
|
|---|---|
| 体外研究 (In Vitro) |
Cefiderocol (S-649266) 是一种与儿茶酚部分缀合的新型肠外铁载体头孢菌素。它对多种需氧革兰氏阴性菌具有很强的抗菌活性,包括铜绿假单胞菌、鲍曼不动杆菌等非发酵菌以及肠杆菌科碳青霉烯类耐药菌株。头孢地考主要与非发酵细菌结合,例如肠杆菌科的 GR20263 和 PBP3。当铜绿假单胞菌中的铁转运蛋白 PiuA 或大肠杆菌中的 CirA 和 Fiu 缺陷时,头孢地罗可的 MIC 可以升高 16 倍,表明这些铁转运蛋白有助于头孢地罗考穿过外膜。铜绿假单胞菌中的外排泵 MexA-MexB-OprM 或肺炎克雷伯菌中的 OmpK35/36 缺陷[1]。
|
| 细胞实验 |
除必须测定特定情况下的 MIC 的情况外,制备贫铁阳离子调节的 Mueller-Hinton 肉汤 (ID-CAMHB) 是为了测定头孢地罗科尔 MIC。头孢菌素对大肠杆菌 ATCC 25922 和铜绿假单胞菌 ATCC 27853 的质量控制最低抑菌浓度 (MIC) 范围为 0.06 至 0.5 μg/mL。补充有血红素、维生素 K1 和湖羊血的布鲁塞氏菌琼脂用于厌氧菌[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A single intravenous dose of 2 g of cefiderocol in healthy patients produces a Cmax of 89.7 mg/L and an AUC of 386 mg\*h/L. In patients with complicated urinary tract infections and a creatinine clearance of at least 60 mL/min, doses of 2 g cefiderocol every 8 hours produced an AUC of 394.7 mg*h/L and a Cmax of 138 mg/L. However the infusion rate for this chronic dosing was 3 times the recommended rate. Cmax and AUC are known to increase proportionally with dosage. 98.6% of cefiderocol is eliminated in the urine with 90.6% as the unchanged parent drug. The remaining 8% is eliminated as metabolites. 2.8% is eliminated in the feces. Less than 10% of cefiderocol is metabolized. Cefiderocol has a mean volume of distribution of 18 L. Cefiderocol has a mean clearance of 5.18 L/h. Metabolism / Metabolites Cefiderocol undergoes a small degree of metabolism to a cefiderocol epimer at the 7 position, cefiderocol catechol-3-methoxy and -4-methoxy, and a pyrrolidine chlorobenzamide product (PCBA). PCBA undergoes further metabolism to sulfated, methylated, and glucuronidated metabolites. The enzymes involved in these reactions have yet to be identified and cefiderocol has not been shown to interfere in the metabolism of other agents. Biological Half-Life The terminal elimination half-life of cefiderocol is 2-3 h. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Although no information is available on the use of cefiderocol during breastfeeding, cephalosporins are generally not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefiderocol is acceptable to use in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Cefiderocol is 40-60% bound to plasma proteins, predominantly to albumin. |
| 参考文献 | |
| 其他信息 |
Cefiderocol is a cephalosporin antibacterial drug and exerts a mechanism of action similar to other β-lactam antibiotics. Unlike other agents in this category, cefiderocol is a siderophore able to undergo active transport into the bacterial cell through iron channels. It represents a significant addition to antibacterial treatment option as it has proven to be effective *in vitro* against multidrug resistant strains including extended spectrum β-lactamase producers and carbapenemase producing bacteria. Cefiderocol was granted designation as a Qualified Infectious Disease Product and granted priority review status by the FDA on November 14, 2019. It is indicated for use in complicated urinary tract infections in patients with limited or no alternative treatments available. This indication was supported by a positive clinical trial composed of 448 patients with complicated urinary tract infections which demonstrated a 72.6% rate of symptom resolution and bacterial eradication with cefiderocol compared to 54.6% with the comparator, imipenem/cilastatin. A concern noted in the trial was a 0.3% higher rate of all cause mortality, the cause of which has not been determined.
Cefiderocol is a Cephalosporin Antibacterial. See also: Cefiderocol Sulfate Tosylate (active moiety of). Drug Indication Cefiderocol is indicated for the treatment of complicated urinary tract infections with or without pyelonephritis. FDA Label Fetcroja is indicated for the treatment of infections  due to aerobic Gram-negative organisms in adults with limited treatment options (see sections 4. 2, 4. 4 and 5. 1). Consideration should be given to official guidance on the appropriate use of antibacterial agents. Treatment of infections due to aerobic Gram-negative bacteria Mechanism of Action Cefiderocol acts by binding to and inhibiting penicillin-binding proteins (PBPs), preventing cell wall synthesis and ultimately causing death of the bacterial cell. Like other β-lactam antibiotics cefiderocol is able to enter bacterial cells via passive diffusion through porins. Unlike other β-lactams, cefiderocol contains a chlorocatechol group which allows it to chelate iron. Once bound to ferric iron cefiderocol is able to undergo active transport into bacterial cells through iron channels in the outer cell membrane such as those encoded by the *cirA* and *fiu* genes in *E. coli* or the *PiuA* gene in *P. aeruginosa*. Once inside the cell, cefiderocol binds to and inhibits PBP3 with high affinity thereby preventing the linking of peptodoglycan layers via the pentapeptide bridge. PBP1a, 1b, 2,and 4 are also bound and inhibited by cefiderocol but with a lesser potency than PBP3 and are therefore expected to contribute less to its antibacterial effect. |
| 分子式 |
C30H34CLN7O10S2
|
|---|---|
| 分子量 |
752.21
|
| 精确质量 |
751.149
|
| 元素分析 |
C, 47.90; H, 4.56; Cl, 4.71; N, 13.03; O, 21.27; S, 8.52
|
| CAS号 |
1225208-94-5
|
| 相关CAS号 |
1883830-01-0 (ditosylate hydrate);1225208-94-5;2009350-94-9 (sulfate tosylate 3:1:4);2135543-94-9 (sulfate tosylate hydrate 3:1:4:1);
|
| PubChem CID |
77843966
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-1.02
|
| tPSA |
310
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
50
|
| 分子复杂度/Complexity |
1440
|
| 定义原子立体中心数目 |
2
|
| SMILES |
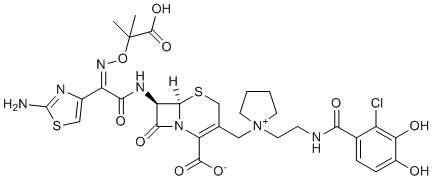
ClC1C(=C(C([H])=C([H])C=1C(N([H])C([H])([H])C([H])([H])[N+]1(C([H])([H])C2C([H])([H])S[C@]3([H])[C@@]([H])(C(N3C=2C(=O)[O-])=O)N([H])C(/C(/C2=C([H])SC(N([H])[H])=N2)=N\OC(C(=O)O[H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O)O[H])O[H]
|
| InChi Key |
DBPPRLRVDVJOCL-FQRUVTKNSA-N
|
| InChi Code |
InChI=1S/C30H34ClN7O10S2/c1-30(2,28(46)47)48-36-19(16-13-50-29(32)34-16)24(42)35-20-25(43)37-21(27(44)45)14(12-49-26(20)37)11-38(8-3-4-9-38)10-7-33-23(41)15-5-6-17(39)22(40)18(15)31/h5-6,13,20,26H,3-4,7-12H2,1-2H3,(H7-,32,33,34,35,36,39,40,41,42,44,45,46,47)/t20-,26-/m1/s1
|
| 化学名 |
(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetamido]-3-({1-[2-(2-chloro-3,4-dihydroxybenzamido)ethyl]pyrrolidin-1-ium-1-yl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
|
| 别名 |
S-649266; S 649266; S649266; GSK2696266D; GSK-2696266D; GSK 2696266D; S-649266D; S 649266D; S649266D; Cefiderocol; Fetroja.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 (2). 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 125 mg/mL (~166.18 mM)
H2O : ~1.06 mg/mL (~1.41 mM) Ethanol : < 1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (3.66 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (3.66 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 27.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (3.66 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.75 mg/mL (3.66 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3294 mL | 6.6471 mL | 13.2942 mL | |
| 5 mM | 0.2659 mL | 1.3294 mL | 2.6588 mL | |
| 10 mM | 0.1329 mL | 0.6647 mL | 1.3294 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|