| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
噻嗪类利尿剂中包括氯噻酮。口服给药后 2-6 小时达到血清峰浓度。氯噻酮的半衰期约为 42(范围 29-55)小时,重复给药后可延长至 45-60 小时。然而,氯噻酮的半衰期因人而异。肾脏以不变的方式消除氯噻酮。氯噻酮的利钠作用在 18 小时达到峰值,并持续超过 48 小时。当比较氯噻酮剂量时,发现每天 25 毫克几乎与更高剂量一样有效,同时低钾血症的风险较低[1]。氢氧化镁不能防止草酸钙结石的复发,而氯噻酮则可以。分析氢氧化镁或氯噻酮是否能更成功地预防草酸钙引起的肾结石复发。采用双盲随机分配设计,每日剂量为 25 或 50 mg。 1,300 或 650 毫克氯噻酮。两种选择之一:氢氧化镁或类似的安慰剂。与治疗前水平相比,所有组的结石事件均显着减少。在整个实验过程中,接受低剂量和高剂量氢氧化镁的组的结石分别减少了 73.9% 和 62.3%(p 分别小于 0.001 和小于 0.01),而安慰剂组的结石比预期减少了 56.1% (p 小于 0.01)。 0.01)。使用氯噻酮治疗使预期发生率降低了 90.1%,并且两种剂量的效果相当。比较治疗时,氯噻酮优于氢氧化镁或安慰剂(p 小于 0.01)。在安慰剂或不积极治疗的情况下观察到的结石事件显着减少,凸显了使用历史对照产生的有利治疗偏差,也强调了使用合适的实验设计的必要性[2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 50% of the administered dose is excreted unmetabolized through the kidney, and excretion is characterized by biphasic elimination with a rapid phase followed by a slow secretory phase. Chlorthalidone has been shown to rapidly concentrate within erythrocytes and subsequently equilibrate via a slow diffusion back into the serum compartment, resulting in a large volume of distribution. BIOCHEM STUDIES SUGGEST THAT PROLONGED DURATION OF ACTION IS DUE TO SLOW GI ABSORPTION & ENTEROHEPATIC RECIRCULATION. DRUG IS EXCRETED UNCHANGED BY KIDNEY. MOST /THIAZIDE/ COMPD ARE RAPIDLY EXCRETED WITHIN 3 TO 6 HR. /THIAZIDE COMPD/ STUDY OF DOSE-DEPENDENT URINARY EXCRETION OF CHLORTHALIDONE. Metabolism / Metabolites Liver Biological Half-Life 40-50 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Chlorthalidone is a thiazide diuretic. Chlorthalidone is indicated in the management of hypertension as sole therapeutic agent or in combination with other antihypertensive drugs. Chlorthalidone is used as adjunctive therapy in the treatment of edema associated with heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. Chlorthalidone has also been found useful in the edema due to various forms of renal dysfunction such as nephrotic syndrome. Chlorthalidone has been used in the treatment of premenstrual tension if there is evidence of fluid retention. Chlorthalidone has a paradoxical antidiuretic effect in patients with diabetes insipidus. HUMAN EXPOSURE: Main risks and target organs: Chlorthalidone is generally well tolerated during therapeutic use. Clinical toxicity is relatively infrequent and may result from overdosage, adverse reactions or hypersensitivity. Acute toxicity: Main risks include: hypokalemia, hyponatremia, hypotension, cardiac arrhythmias and central nervous system effects. Target organs: kidney, heart, CNS. Chronic toxicity and adverse reactions include: Metabolic disturbances, hypersensitivity reactions, aggravation of renal and/or hepatic insufficiency, gastrointestinal disturbances, blood dyscrasias, and central nervous system effects. Summary of clinical effects: Acute toxicity: Symptoms may include: hypokalemia, hyponatremia, dehydration, hypovolemic, cardiac dysrhythmias (ventricular extrasystoles and torsade de pointes due to hypokalemia), dizziness, lethargy, paresthesias. Chronic toxicity and adverse reactions: Several disturbances have been reported: Metabolic: hypokalemia, hyponatremia, hyperglycemia, hyperuricemia, metabolic alkalosis, aggravation of renal insufficiency. Cardiovascular: cardiac arrhythmias, enhancing the effect of digitalis on cardiac muscle, orthostatic hypotension. Central Nervous System: dizziness, vertigo, paresthesias, headache, xanthopsia. Gastrointestinal: anorexia, gastric irritation, nausea, vomiting, cramping, diarrhea, constipation, jaundice due to intrahepatic cholestasis, pancreatitis, sialoadenitis, dry mouth, hepatic insufficiency, intestinal ulceration. Hypersensitivity: purpura, intravascular immune hemolysis, pneumonitis, skin rashes, urticaria, eczema, lichen planus like reactions; photosensitivity, similar to subacute cutaneous lupus erythematosus; vasculitis; Stevens Johnson Syndrome. Hematological: thrombocytopenia, granulocytopenia, leucopenia, aplastic anemia, and hemolytic anemia. Respiratory tract: acute noncardiogenic pulmonary edema. Others: attacks of gout, increasing in plasma concentrations of cholesterol and triglycerides. Use: Chlorthalidone may prevent renal calculus formation in patients with hypercalciuria. Chlorthalidone may improve vertigo associated with Ménière disease. Contraindications: Anuria, hypersensitivity to sulfonamide-derived drugs, hepatic encephalopathy. Precautions: Chlorthalidone should be used with caution in: impaired hepatic function since it may increase the risk of hepatic encephalopathy. Renal impairment can occur since it can further reduce renal function, and precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function. Patients treated with quinidine-like anti-arrhythmics, amiodarone, sotalol. Avoid use in patients with gout since it can precipitate attacks of the disease. Patients should be carefully observed for signs of fluids and electrolyte imbalance. Chlorthalidone may enhance the toxicity of digitalis glycosides by depleting serum potassium concentrations. The possibility of exacerbation or precipitation of systemic lupus erythematosus has been reported. Chlorthalidone crosses the placenta and there have been reports of neonatal jaundice, thrombocytopenia, and electrolytes imbalances following maternal treatment. Chlorthalidone is excreted in breast milk. Treatment can inhibit lactation. Chlorthalidone should be used with caution when the following medical problems exist: diabetes mellitus, hypercalcemia, hyperuricemia, history of lupus erythematosus, pancreatitis, sympathectomy. Routes of entry: The oral route is the commonest route of administration. Accidental or deliberate ingestion of large doses may occur. Kinetics: Absorption by route of exposure: Chlorthalidone is erratically absorbed from the gastrointestinal tract. Bioavailability after oral administration is approximately 65%. About 75% is bound to plasma protein and the blood-to-plasma ratio is 72.5%. Chlorthalidone crosses the placenta. Biological half-life by route of exposure: The plasma half-life is about 44 +/- 10 hours and increases with age. The terminal half-life is 35 to 54 hours. This may be due to the strong binding of chlorthalidone to red blood cells. Metabolism: Chlorthalidone probably undergoes metabolism but the metabolites have not been identified. Elimination by route of exposure: During long-term administration, 30 to 60% has been reported to be excreted unchanged in the urine. Chlorthalidone is excreted in breast milk. It has a very low milk:plasma ratio. Chlorthalidone is an oral diuretic with prolonged action and low toxicity. Most of the toxic effects are due to electrolyte imbalance including hypochloremic alkalosis, hyponatremia, hypokalemia, and hypomagnesemia. The mechanism of hypercalcemia and hypophosphatemia are unknown. Thiazides increase the concentration of cholesterol and triglycerides in plasma. Chlorthalidone may also cause hypersensitivity reactions. Pharmacodynamics: The diuretic effect of the drug occurs within two hours after an oral dose and continues for up to 72 hours. It produces copious loss of electrolytes, and consequently, of water. The site of action is the distal renal tubule. The hypotensive effect is also due to a reduction in peripheral resistance observed mainly in chronic use. Teratogenicity: Chlorthalidone crosses the placental barrier. In general, diuretics are not associated with teratogenicity. A slight association with respiratory malformation has been suggested. Interactions: Chlorthalidone may increase the toxicity of digitalis glycosides by depleting serum potassium concentrations. Due to the potassium depletion, chlorthalidone may enhance the neuromuscular blocking action of competitive muscle relaxants such as tubocurarine or gallamine triethiodide. It may increase the effect of antihypertensive agents such as guanethidine sulfate, methyldopa or ganglionic blocking agents. The postural hypotension due to thiazide diuretic therapy may be increased by concomitant ingestion of alcohol, barbiturates, or opioids. The potassium-depleting effect of thiazides may be enhanced by corticosteroids, corticotrophin, carbenoxolone, and amphotericin B. Chlorthalidone may reduce the response to pressor amines such as norepinephrine, but the clinical significance of this effect is uncertain. Concomitant administration of thiazide diuretics and lithium salts is not recommended since the blood concentration of lithium is increased. By depleting the serum potassium concentration, chlorthalidone may increase the risk of cardiac dysryhthmias (torsades de pointes). The pharmacological effects of oral hypoglycaemic agents may be reduced. Non-steroidal anti-inflammatory drugs may antagonize the diuretic actions of thiazides. The hyperglycemic, hypotensive, and hyperuricaemic effects of diazoxide may be potentiated by thiazides. Probenecid enhances excretion of calcium, magnesium, and citrate during thiazide therapy, but does not affect excretion of sodium, potassium, ammonium chloride, bicarbonate, and phosphate. Thiazides increase urinary pH and may decrease urinary excretion of amphetamines and quinidine. The effects of oral anticoagulants may be decreased when used concurrently with chlorthalidone. Pre-anesthetic and anesthetic drugs used in surgery may be potentiated when used concurrently with chlorthalidone. The effectiveness of methenamine may be decreased when used with chlorthalidone due to alkalinization of the urine. A study in two healthy subjects evidenced that chlorthalidone and acetazolamide competed for the same binding sites on blood cells. Main adverse effects: It may induce hyperglycemia and glycosuria and may aggravate pre-existing diabetes mellitus. Other side-effects include anorexia, gastric irritation, nausea, vomiting, constipation, diarrhea, headache, dizziness, postural hypotension, paraesthesia, impotence, mood and mental changes and yellow vision. Blood dyscrasias include thrombocytopenia, more rarely, granulocytopenia, leucopenia and aplastic anemia. administration of tablets containing thiazides with an enteric-coated core of potassium chloride. Chlorthalidone may cause intense diuresis leading to insomnia in the elderly, because of its long half-life. ANIMAL/PLANT STUDIES: Reproduction studies conducted with rats and rabbits at doses up to 240 times greater than the therapeutic dose have shown no evidence of impaired fertility or harm to the fetus due to chlorthalidone. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although amounts of chlorthalidone in milk are not great, its slow clearance may lead to accumulation in the infant, especially while nursing a newborn or preterm infant. It may also suppress lactation. An alternate drug may be preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Chlorthalidone has been used successfully to suppress lactation by giving 200 mg orally right after delivery, followed by 100 mg daily for 3 days in conjunction with fluid restriction and breast binding. However, a comparative study found no difference between chlorthalidone 200 mg daily for 7.6 days and placebo in milk leakage and breast engorgement and pain. The added contribution of the diuretic to fluid restriction and breast binding, which are effective in suppressing lactation, has not been studied. There are no data on the effects of diuretics on established, ongoing lactation. Protein Binding Approximately 75 percent of the drug is bound to plasma proteins, 58 percent of the drug being bound to albumin. This is caused by an increased affinity of the drug to erythrocyte carbonic anhydrase. Interactions CHLOROTHIAZIDE & OTHER THIAZIDE DIURETICS /INCL CHLORTHALIDONE/ ENHANCE CARDIOTOXIC & NEUROTOXIC EFFECTS OF LITHIUM... CHLORTHALIDONE.../IS/ STRUCTURALLY RELATED TO THIAZIDE DIURETICS...SHOULD BE EXPECTED TO INTERACT WITH GUANETHIDINE /ENHANCING ITS HYPERTENSIVE ACTION/... /CHLORTHALIDONE/...MAY...INTERACT WITH PROBENECID /ANTAGONIZING ITS URICOSURIC EFFECTS & CAUSING URIC ACID RETENTION/. Concurrent use of thiazide diuretics with amiodarone may lead to an increased risk of arrhythmias associated with hypokalemia. /Thiazide diuretics/ For more Interactions (Complete) data for CHLORTHALIDONE (17 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Barrios V, et al. Which thiazide to choose as add-on therapy for hypertension? Integr Blood Press Control. 2014 Jul 30;7:35-47.
[2]. Ettinger B, et al. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988 Apr;139(4):679-84. |
| 其他信息 |
Therapeutic Uses
Antihypertensive Agents; Diuretics, Sulfamyl ...ORALLY EFFECTIVE DIURETIC USEFUL IN TREATMENT OF EDEMA ASSOC WITH CONGESTIVE HEART FAILURE, RENAL DISEASE, HEPATIC CIRRHOSIS, PREGNANCY, OBESITY, & PREMENSTRUAL SYNDROME. DIURETIC EFFECTS START WITHIN 2 HR AFTER ADMIN, REACH PEAK IN 6 HR, & PERSIST FOR 48 TO 72 HR. MOST OF THIAZIDES ARE GIVEN IN DIVIDED DAILY DOSES FOR TREATMENT OF HYPERTENSION, BUT SINGLE DAILY DOSE MAY BE PREFERABLE FOR MOBILIZATION OF EDEMA FLUID. ...CHLORTHALIDONE...SHOULD BE GIVEN LESS FREQUENTLY, SINCE.../IT HAS/ DURATION OF ACTION LONGER THAN 24 HR. CHLORTHALIDONE ALSO EXERTS ANTIHYPERTENSIVE EFFECT & MAY BE ADMIN WITH OTHER AGENTS, SUCH AS RESERPINE, GANGLIONIC BLOCKING AGENTS, HYDRALAZINE, & GUANETHIDINE. SINCE.../IT/ CONTAINS SULFONAMIDE GROUP, ITS PHARMACOLOGICAL ACTIONS & MANY OF ITS UNTOWARD EFFECTS ARE SIMILAR TO THOSE OF OTHER ORALLY ADMIN DIURETICS. For more Therapeutic Uses (Complete) data for CHLORTHALIDONE (11 total), please visit the HSDB record page. Drug Warnings CHLORTHALIDONE IS CONTRAINDICATED IN PT WITH SEVERE RENAL OR HEPATIC DISEASE. PT ON THIS DRUG SHOULD BE WATCHED CLOSELY FOR SYMPTOMS OF RENAL DAMAGE OR OF ELECTROLYTE DISTURBANCE. May suppress lactation ... /Thiazide diuretics; from table/ Many experts consider diuretics contraindicated in pregnancy except for patients with heart disease, since they do not prevent or alter course of toxemia and may decrease placental perfusion. /Chlorothiazide; from table/ Maternal Medication usually Compatible with Breast-Feeding: Chlorthalidone: Reported Sign or Symptom in Infant or Effect on Lactation: Excreted slowly. /from Table 6/ For more Drug Warnings (Complete) data for CHLORTHALIDONE (14 total), please visit the HSDB record page. |
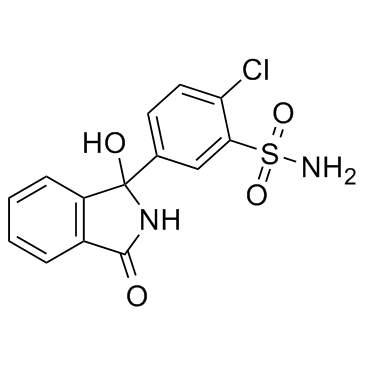
| 分子式 |
C14H11CLN2O4S
|
|---|---|
| 分子量 |
338.76
|
| 精确质量 |
338.012
|
| CAS号 |
77-36-1
|
| 相关CAS号 |
Chlorthalidone-d4;1794941-44-8
|
| PubChem CID |
2732
|
| 外观&性状 |
Crystals from 50% acetic acid
WHITE TO YELLOWISH-WHITE CRYSTALLINE POWDER |
| 密度 |
1.6±0.1 g/cm3
|
| 熔点 |
265-267ºC (dec.)
|
| 折射率 |
1.694
|
| LogP |
-0.74
|
| tPSA |
117.87
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
564
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1S(N([H])[H])(=O)=O)C1(C2=C([H])C([H])=C([H])C([H])=C2C(N1[H])=O)O[H]
|
| InChi Key |
JIVPVXMEBJLZRO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H11ClN2O4S/c15-11-6-5-8(7-12(11)22(16,20)21)14(19)10-4-2-1-3-9(10)13(18)17-14/h1-7,19H,(H,17,18)(H2,16,20,21)
|
| 化学名 |
2-chloro-5-(1-hydroxy-3-oxo-2H-isoindol-1-yl)benzenesulfonamide
|
| 别名 |
Thalitone Chlorthalidone Chlorphthalidolone Phthalamodine OxodolineChlortalidone Phthalamudine Hygroton Phthalamudine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 41 mg/mL (~121.03 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.14 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9519 mL | 14.7597 mL | 29.5194 mL | |
| 5 mM | 0.5904 mL | 2.9519 mL | 5.9039 mL | |
| 10 mM | 0.2952 mL | 1.4760 mL | 2.9519 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Diuretic Use in Hemodialysis Patients With Residual Renal Function
CTID: NCT05915286
Phase: Phase 4 Status: Suspended
Date: 2024-03-05
|