| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
通过 C21 位点的酯裂解,母体分子环索奈德被水解为活性代谢物去异丁酰环索奈德 (des-CIC),随后在肺细胞中可逆地产生脂肪酸酯。正常人支气管上皮 (NHBE) 细胞快速水解 cleisonide (5 μM)(4 小时转化率约为 30%),24 小时几乎完全转化 [1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ciclesonide and des-ciclesonide have negligible oral bioavailability (both less than 1%) due to low gastrointestinal absorption and high first-pass metabolism. The intranasal administration of ciclesonide at recommended doses results in negligible serum concentrations of ciclesonide. 152 L/hr [Following IV administration of 800 mcg of ciclesonide] Metabolism / Metabolites Des-ciclesonide undergoes metabolism in the liver to additional metabolites mainly by the cytochrome P450 (CYP) 3A4 isozyme and to a lesser extent by CYP 2D6. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Although not measured, the amounts of inhaled corticosteroids absorbed into the maternal bloodstream and excreted into breastmilk are probably too small to affect a breastfed infant. Expert opinion considers inhaled, nasal and oral corticosteroids acceptable to use during breastfeeding. ◉ Effects in Breastfed Infants None reported with any corticosteroid. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The percentage of ciclesonide and des-ciclesonide bound to human plasma proteins averaged ≥ 99% each, with ≤ 1% of unbound drug detected in the systemic circulation. |
| 参考文献 | |
| 其他信息 |
Ciclesonide is an organic molecular entity.
Ciclesonide is a glucocorticoid used to treat obstructive airway diseases. It is marketed under the brand name Alvesco. Ciclesonide is a nonhalogenated, synthetic, inhaled corticosteroid (ICS) with anti-inflammatory and potential antiviral activities. Upon administration by oral inhalation into the lungs, ciclesonide (CIC) is converted by local esterases to its active metabolite desisobutyryl-ciclesonide (des-CIC), which binds to intracellular glucocorticoid receptors (GRs). The ligand-bound GRs regulate gene expressions, which lead to inhibitory activities against multiple cell types, such as mast cells, eosinophils, basophils, lymphocytes, macrophages and neutrophils, and various mediators associated with inflammation, such as histamine, eicosanoids, leukotrienes and cytokines. In addition, ciclesonide may suppress the replication of human coronavirus by targeting viral nonstructural protein 15 (NSP15). Drug Indication For the treatment of nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and adolescents 12 years of age and older. For the alleviation of clinical signs of severe equine asthma (formerly known as Recurrent Airway Obstruction â (RAO), Summer Pasture Associated Recurrent Airway Obstruction â (SPA-RAO)). Mechanism of Action Glucocorticoids such as ciclesonide can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses. The antiinflammatory actions of glucocorticoids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes. Ciclesonide reduces inflammatory reaction by limiting the capillary dilatation and permeability of the vascular structures. These compounds restrict the accumulation of polymorphonuclear leukocytes and macrophages and reduce the release of vasoactive kinins. Recent research suggests that corticosteroids may inhibit the release of arachidonic acid from phospholipids, thereby reducing the formation of prostaglandins. Ciclesonide is a glucocorticoid receptor agonist. On binding, the corticoreceptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing an increase or decrease in expression of specific target genes, including suppression of IL2 (interleukin 2) expression. Pharmacodynamics Ciclesonide is a pro-drug that is enzymatically hydrolyzed to a pharmacologically active metabolite, C21-desisobutyryl-ciclesonide (des-ciclesonide or RM1) following intranasal application. Des-ciclesonide has anti-inflammatory activity with affinity for the glucocorticoid receptor that is 120 times higher than the parent compound. The precise mechanism through which ciclesonide affects allergic rhinitis symptoms is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic inflammation. |
| 分子式 |
C32H44O7
|
|---|---|
| 分子量 |
540.6876
|
| 精确质量 |
540.309
|
| CAS号 |
126544-47-6
|
| 相关CAS号 |
Desisobutyryl-ciclesonide;161115-59-9;Ciclesonide (Standard);126544-47-6;Ciclesonide-d7;1225382-70-6
|
| PubChem CID |
6918155
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.23 g/cm3
|
| 沸点 |
665ºC at 760 mmHg
|
| 熔点 |
202-209?C
|
| 闪点 |
210ºC
|
| 蒸汽压 |
1.61E-20mmHg at 25°C
|
| 折射率 |
1.575
|
| LogP |
4.703
|
| tPSA |
99.13
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
1100
|
| 定义原子立体中心数目 |
9
|
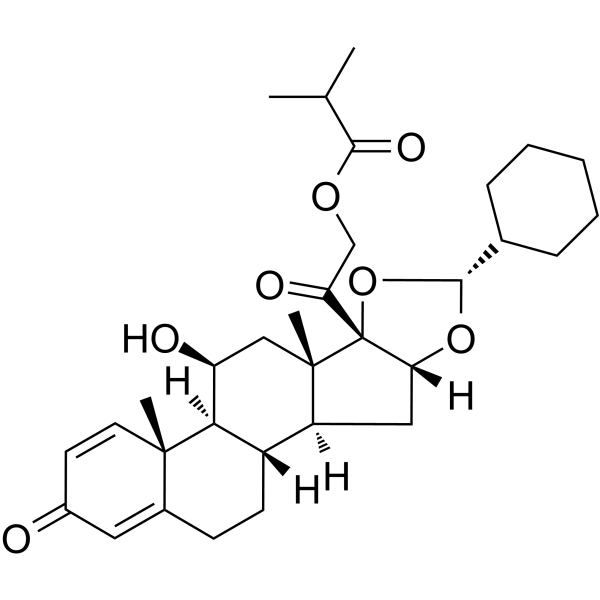
| SMILES |
O1[C@]([H])(C2([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C2([H])[H])O[C@]2([H])C([H])([H])[C@@]3([H])[C@]4([H])C([H])([H])C([H])([H])C5=C([H])C(C([H])=C([H])[C@]5(C([H])([H])[H])[C@@]4([H])[C@]([H])(C([H])([H])[C@]3(C([H])([H])[H])[C@]12C(C([H])([H])OC(C([H])(C([H])([H])[H])C([H])([H])[H])=O)=O)O[H])=O
|
| InChi Key |
LUKZNWIVRBCLON-GXOBDPJESA-N
|
| InChi Code |
InChI=1S/C32H44O7/c1-18(2)28(36)37-17-25(35)32-26(38-29(39-32)19-8-6-5-7-9-19)15-23-22-11-10-20-14-21(33)12-13-30(20,3)27(22)24(34)16-31(23,32)4/h12-14,18-19,22-24,26-27,29,34H,5-11,15-17H2,1-4H3/t22-,23-,24-,26+,27+,29+,30-,31-,32+/m0/s1
|
| 化学名 |
[2-[(1S,2S,4R,6R,8S,9S,11S,12S,13R)-6-cyclohexyl-11-hydroxy-9,13-dimethyl-16-oxo-5,7-dioxapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-8-yl]-2-oxoethyl] 2-methylpropanoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~92.47 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8495 mL | 9.2474 mL | 18.4949 mL | |
| 5 mM | 0.3699 mL | 1.8495 mL | 3.6990 mL | |
| 10 mM | 0.1849 mL | 0.9247 mL | 1.8495 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The Mannitol-Asthma-Ciclesonide-Study
CTID: NCT03839433
Phase: Phase 4 Status: Completed
Date: 2019-07-25