| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:Ciclopirox olamine (CPX) 是一种亲脂性二齿铁螯合剂,在常氧条件下以比其他铁螯合剂更低的浓度稳定 HIF-1α,可能是通过抑制 HIF-1α 羟基化来实现的。环吡酮乙醇胺 (CPX) 诱导的 HIF-1 介导报告基因活性和内源性 HIF-1 靶基因表达,包括血管内皮生长因子 (VEGF) 转录、mRNA 和蛋白质水平的升高。 Ciclopirox 以剂量依赖性方式抑制白色念珠菌酵母和菌丝细胞的生长。环吡酮通过维持线粒体跨膜电位 (Deltapsim) 来阻断 H2O2 诱导的线粒体损伤。在腺癌 SK-HEP-1 细胞中,Ciclopirox 完全阻断 H2O2 刺激的乳酸脱氢酶(细胞死亡标记)的释放,并减少 MTT 减少(线粒体功能标记)。 Ciclopirox 有效抑制 H2O2 诱导的线粒体通透性转换孔 (MPTP) 打开。在葡萄糖剥夺的 SIN-1 处理的星形胶质细胞中,Ciclopirox 会增加 MTP,将其维持在高水平,并阻止 ATP 消耗。环吡酮通过减轻过氧亚硝酸盐诱导的线粒体功能障碍来保护星形胶质细胞免受过氧亚硝酸盐细胞毒性。环吡酮是一种取代的吡啶酮抗真菌药物,与咪唑衍生物无关,其局部应用可确保最大的局部生物利用度。环吡酮通过抑制细胞内必需底物和离子的摄取来作用于真菌,这可能作用于念珠菌表达其粘附机制的能力。细胞测定:沙氏葡萄糖培养基(2%)用于细胞培养生长,RPMI 2%葡萄糖培养基和2%沙氏葡萄糖培养基用于MIC测定。对于细胞培养物生长曲线,以105个细胞/mL接种220mL含有不同浓度环吡酮的2%沙氏葡萄糖培养基,并将混合物在160rpm和37℃下振荡1-10小时。在 630 nm 处用光度法测量生长情况。将不同浓度的 FeCl3 或 2,2-联吡啶添加到培养基中进行抑制研究。
|
|---|---|
| 体内研究 (In Vivo) |
采用小鼠皮肤创伤模型、大鼠肾脏模型、鸡绒毛尿囊膜模型等不同动物器官模型研究环吡酮对内源性HIF-1靶基因VEGF的影响。结果显示,CPX 功能性激活 HIF-1,诱导 VEGF 表达并加速血管生成。
|
| 细胞实验 |
沙氏葡萄糖培养基(2%)用于细胞培养生长,RPMI 2%葡萄糖培养基和2%沙氏葡萄糖培养基用于MIC测定。对于细胞培养物生长曲线,以105个细胞/mL接种220mL含有不同浓度环吡酮的2%沙氏葡萄糖培养基,并将混合物在160rpm和37℃下振荡1-10小时。在 630 nm 处通过光度法测量生长情况。将不同浓度的 FeCl3 或 2,2-联吡啶添加到培养基中进行抑制研究。
|
| 动物实验 |
Different animal organ models including mouse skin wound model, rat kidney model and chicken chorioallantoic membrane model
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed after oral administration. Mean absorption of ciclopirox after application to nails of all twenty digits and adjacent 5 millimeters of skin once daily for 6 months in patients with dermatophytic onychomycoses was less than 5% of the applied dose. Ciclopirox olamine also penetrates into hair and through the epidermis and hair follicles into sebaceous glands and dermis. Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides. Metabolism / Metabolites Glucuronidation is the main metabolic pathway of ciclopirox. Glucuronidation is the main metabolic pathway of ciclopirox. Route of Elimination: Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides. Half Life: 1.7 hours for 1% topical solution. Biological Half-Life 1.7 hours for 1% topical solution. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Topical ciclopirox has not been studied during breastfeeding. Because only about 1.3% is absorbed after topical application, it is considered a low risk to the nursing infant.[1] Avoid application to the nipple area and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[2] ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Protein binding is 94-97% following topical administration. |
| 参考文献 | |
| 其他信息 |
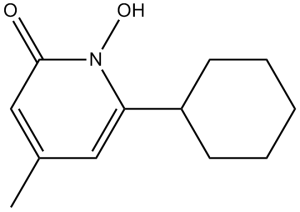
Ciclopirox is a cyclic hydroxamic acid that is 1-hydroxypyridin-2(1H)-one in which the hydrogens at positions 4 and 6 are substituted by methyl and cyclohexyl groups, respectively. A broad spectrum antigfungal agent, it also exhibits antibacterial activity against many Gram-positive and Gram-negative bacteria, and has anti-inflammatory properties. It is used a a topical treatment of fungal skin and nail infections. It has a role as an antibacterial agent and an antiseborrheic. It is a pyridone, a cyclic hydroxamic acid and a hydroxypyridone antifungal drug.
Ciclopirox olamine (used in preparations called Batrafen, Loprox, Mycoster, Penlac and Stieprox) is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. In particular, the agent is especially effective in treating Tinea versicolor. The mechanism of action of ciclopirox is as a Protein Synthesis Inhibitor. The physiologic effect of ciclopirox is by means of Decreased DNA Replication, and Decreased Protein Synthesis, and Decreased RNA Replication. Ciclopirox is a synthetic, broad-spectrum antifungal agent with additional antibacterial and anti-inflammatory activities. Ciclopirox exerts its action by binding to and chelating trivalent cations, such as Fe3+ and Al3+, thereby inhibiting the availability of essential co-factors for enzymes. This may lead to a loss of activity of enzymes that are essential for cellular metabolism, organization of cell wall structure and other crucial cell functions. In addition, ciclopirox exerts its anti-inflammatory activity by inhibiting 5-lipoxygenase and cyclooxygenase (COX). Ciclopirox is only found in individuals that have used or taken this drug. It is a synthetic antifungal agent for topical dermatologic use. [Wikipedia] Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase. Ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport. A cyclohexane and pyridinone derivative that is used for the treatment of fungal infections of the skin and nails, and for treatment of VAGINAL YEAST INFECTIONS. See also: Ciclopirox Olamine (has salt form); Ciclopirox; clobetasol propionate (component of); Ciclopirox; fluconazole; terbinafine (component of). Drug Indication Used as a topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum. FDA Label Mechanism of Action Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase. ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport. |
| 分子式 |
C12H17NO2
|
|
|---|---|---|
| 分子量 |
207.27
|
|
| 精确质量 |
207.125
|
|
| 元素分析 |
C, 69.54; H, 8.27; N, 6.76; O, 15.44
|
|
| CAS号 |
29342-05-0
|
|
| 相关CAS号 |
Ciclopirox olamine;41621-49-2;Ciclopirox olamine;41621-49-2;Ciclopirox-d11;Ciclopirox-d11 sodium
|
|
| PubChem CID |
2749
|
|
| 外观&性状 |
White to off-white solid powder.
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
350.0±25.0 °C at 760 mmHg
|
|
| 熔点 |
1440C
|
|
| 闪点 |
165.5±23.2 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.582
|
|
| LogP |
2.59
|
|
| tPSA |
42.23
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
15
|
|
| 分子复杂度/Complexity |
325
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O([H])N1C(C([H])=C(C([H])([H])[H])C([H])=C1C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O
|
|
| InChi Key |
SCKYRAXSEDYPSA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3
|
|
| 化学名 |
6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 41~100 mg/mL ( 197.8~482.46 mM )
Ethanol : 41 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.06 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.06 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (12.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + Corn oil: 3mg/ml (14.47mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8246 mL | 24.1231 mL | 48.2462 mL | |
| 5 mM | 0.9649 mL | 4.8246 mL | 9.6492 mL | |
| 10 mM | 0.4825 mL | 2.4123 mL | 4.8246 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05809297 | Not yet recruiting | Drug: Ciclopirox Hydroxypropyl Chitosan (HPCH) Nail Lacquer |
Onychomycosis | Universidad Complutense de Madrid | September 1, 2023 | Phase 4 |
| NCT02679911 | Completed Has Results |
Drug: Loceryl NL Drug: Ciclopirox NL |
Foot Dermatoses | Galderma R&D | September 2015 | Phase 4 |
| NCT00990587 | Completed | Drug: Ciclopirox Olamine | Hematologic Malignancy Acute Lymphocytic Leukemia |
University Health Network, Toronto | October 2009 | Phase 1 |
| NCT01646580 | Terminated | Drug: ciclopirox | Dermatomycoses | Ferrer Internacional S.A. | October 2008 | Phase 4 |