| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
DNA Alkylator/Crosslinker
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:顺铂是一种无机铂络合物,是一种化疗剂,也是多种细胞类型生长停滞和凋亡的有效诱导剂。它用于治疗多种癌症,包括睾丸癌、卵巢癌、宫颈癌、乳腺癌、膀胱癌、头颈癌、食道癌、肺癌、间皮瘤、脑肿瘤和神经母细胞瘤。 IV(静脉内)给药后,顺铂形成高反应性、带电荷的铂络合物,该络合物与 DNA 中富含 GC 的位点等亲核基团结合,诱导链内和链间 DNA 交联,以及 DNA-蛋白质交联。这会抑制 DNA 合成并导致细胞凋亡和细胞生长抑制。顺铂通过与 DNA 相互作用形成 DNA 加合物来诱导细胞毒性,从而激活多种信号转导途径,包括 Erk、p53、p73 和 MAPK,最终导致细胞凋亡的激活。顺铂 (30 mM) 处理 6 小时可诱导 HeLa 细胞中 Erk 明显激活,并在接下来的 14 小时内持续。顺铂还通过诱导肿瘤细胞死亡而显示出有效的抗肿瘤活性。顺铂表现出引起肾近端肾小管细胞 (RPTC) 凋亡的能力,导致细胞收缩、半胱天冬酶 3 活性增加 50 倍、磷脂酰丝氨酸外化增加 4 倍、染色质浓缩和 DNA 增加 5 倍和 15 倍分别为亚倍体。顺铂 (800 μM) 治疗 4 小时后会导致 RPTC 坏死的典型特征。细胞测定:L1210/0 细胞在补充有 15% 小牛血清和 Fungizone 的 McCoys 培养基 5a(改良)中于 37℃、5% CO2 潮湿气氛中维持指数悬浮培养。 L1210/0 细胞在顺铂 (7 μg/mL) 中于 37 ℃ 孵育 2 小时。为了测量生长抑制,将细胞离心,洗涤一次,以 30 × 103 至 50 × 103 细胞/mL 重悬于新鲜培养基中,并孵育 3 天。细胞数在库尔特计数器上测定。将等份细胞用等体积的 0.4% 台盼蓝稀释。活力记录为排除台盼蓝的细胞的百分比。将如上所述与顺铂一起孵育的细胞也稀释到 0.1% 琼脂中,并在对集落进行计数时使其生长 2 周。
|
| 体内研究 (In Vivo) |
在携带黑色素瘤的小鼠中,顺铂(4 mg/kg B.W.)减小了实体瘤的大小和重量,补充顺铂的HemoHIM增强了肿瘤大小(p<0.01)和重量(p<0.01)的减小。HemoHIM本身在体外不抑制黑色素瘤细胞生长,也不干扰顺铂的体外作用。然而,HemoHIM给药增强了小鼠的NK细胞和Tc细胞活性。有趣的是,HemoHIM增加了脾脏中NK细胞的比例。在用顺铂治疗的携带黑色素瘤的小鼠中,HemoHIM给药还增加了NK细胞和Tc细胞的活性以及脾细胞分泌的IL-2和IFN-γ,这似乎有助于HemoHIM增强顺铂的疗效。此外,HemoHIM还降低了肾小管细胞破坏的肾毒性。
结论:HemoHIM可能是顺铂化疗期间的有益补充,可增强顺铂的抗肿瘤疗效,降低顺铂的毒性。[8]
研究了三种不同来源的人癌症异种移植物和作为成熟肿瘤在裸鼠体内经皮下生长的移植物对Cisplatin/顺铂(CDDP)、环磷酰胺(CTX)、131I-标记的抗表生蛋白单克隆抗体(MAb)139H2或体外放射治疗的敏感性。每周静脉注射CDDP的最大耐受剂量x 2可诱导77.5%的肿瘤生长抑制(GI)和85.1%的浆液性异种移植物Ov。Ri(C)和OVCAR-3。Ov粘液异种移植物。Pe对CDDP具有相对抗性。CTX的最大耐受剂量,以2周的间隔腹腔注射2次,诱导3种异种移植物中每种移植物的GI在52.9%至59.7%之间。用500-750 microCi 131I特异性MAb 139H2进行放射免疫治疗,间隔2周静脉注射x 2,比CDDP或CTX更有效。500微Ci 131I MAb 139H2方案诱导Ov 100%GI。Ri(C)异种移植物和所有肿瘤均治愈。同样的方案在OVCAR-3异种移植物中的效果稍差,但仍然可以获得完全的肿瘤消退。Ov。Pe异种移植物对放射免疫治疗最不敏感。两次注射500 microCi 131I对照MAb仅对OVCAR-3和Ov产生短暂的生长抑制。Pe肿瘤,但Ov完全消退。Ri(C)异种移植物。使用131I MAb 139H2和125I对照MAb的示踪剂剂量进行生物分布,在3种异种移植物中对MAb 139H 2显示出不同程度的特异性。OV吸收的辐射剂量。Ri(C)、OVCAR-3和Ov。每10 microCi注射剂量的Pe异种移植物分别为30、41和29cGy。10 Gy外照射治疗表明,每种肿瘤系的放射免疫治疗效果与异种移植物的内在放射敏感性有关[6]。 顺铂/Cisplatin已被证明可有效抑制多种动物肿瘤模型中的肿瘤生长,包括头颈癌异种移植物、宫颈鳞状细胞癌异种移植物、睾丸癌异种移植物、卵巢癌异种移植物、乳腺癌异种移植物、结肠癌、异种移植肝母细胞瘤、等等。在第 1 天和第 7 天每周静脉注射顺铂 (5 mg/kg),可分别诱导浆液性异种移植物 Ov.Ri(C) 和 OVCAR-3 77.5% 和 85.1% 的肿瘤生长抑制 (GI)。 顺铂诱导的急性肾损伤(CI-AKI)小鼠模型[9] 按照文献报道方法顺铂诱导的急性肾损伤模型(Kim等人,2012)。简而言之,在诱导前,8-12周龄的雄性C57BL/6小鼠被剥夺食物和水18小时。 仅使用雄性小鼠,因为雌性小鼠对肾损伤更具抵抗力(Müller等人,2002;Wei等人,2005;Yang等人,2010)。顺铂通过以1mg/ml的浓度溶解在无菌生理盐水中,然后在37°C水浴中孵育30分钟来制备注射用顺铂,以确保溶解,同时避光。然后给小鼠腹腔注射25mg/kg,此时归还食物和水。在晚期眼眶后出血后注射顺铂72小时后处死小鼠。除了血清采集外,还采集了肾脏并准备进行进一步研究。健康对照组要么禁食脱水,不注射顺铂,要么随意进食和饮水。 |
| 细胞实验 |
L1210/0 细胞在补充有 15% 小牛血清和 Fungizone 的 McCoy 培养基 5a(改良)中,在 37 °C、5% CO2 潮湿气氛中进行指数悬浮培养。将 L1210/0 细胞在 7 μg/mL 顺铂中在 37°C 下孵育两小时。将细胞离心,再次清洗,以 30 × 103 至 50 × 103 细胞/mL 重悬于新鲜培养基中,孵育三天以进行测量生长抑制。库尔特计数器用于计算细胞数。将台盼蓝(0.4% 体积)添加到等份细胞中进行稀释。不含台盼蓝的细胞百分比用于确定活力。如前所述,用顺铂培养的细胞生长两周后对集落进行计数,然后将其稀释到 0.1% 琼脂中。
|
| 动物实验 |
Cisplatin injection and HemoHIM administration in tumor-bearing mice model [8]
Mice were divided randomly into three groups (Control, Cisplatin and Cisplatin+HemoHIM), and each group consisted of twenty mice. B16F0 melanoma (5 × 105 cells/mouse) was inoculated into subcutaneous femoral left region of mice at 3 days before an initial injection of cisplatin. Cisplatin was injected intraperitoneally at 4 mg/kg body weight (B.W.) on day 0, 7 and 14 (total three injections). Experimental group was intubated with HemoHIM at a final concentration of 100 mg/kgB.W. by everyday from day -1 to day 16, while the control group received only water. The scheme of the administration procedure is summarized in Fig. 1. On day 17 after initial injection of cisplatin, all mice of each group were experimented, respectively, to evaluate tumor weight or tumor size. The tumor size was calculated as follows: tumor size = ab2/2, where a and b are the larger and smaller diameters, respectively. Mice: There are twenty mice per group, which are randomly assigned to three groups: Control, Cisplatin, and Cisplatin+HemoHIM. A subcutaneous femoral left region in mice is injected with B16F0 melanoma (5×105 cells/mouse) three days prior to the first Cisplatin injection. Three injections of 4 mg/kg body weight (B.W.) of Cisplatin are administered intraperitoneally on days 0 through 14. Day 0 to Day 16 see daily intubations of the experimental group with HemoHIM at a final concentration of 100 mg/kgB.W., while the control group was given only water. Each group's mice undergo experiments on day 17 following their first Cisplatin injection in order to assess the tumors' size or weight. The tumor size is calculated as follows: tumor size=ab2/2, where a and b are the larger and smaller diameters, respectively. Rats: Four groups of four or five male Sprague-Dawley rats, weighing 200 to 250 g apiece, are randomly assigned. First, a vehicle containing 5% carboxymethyl cellulose sodium solution (CMC-Na), 5 mL/kg body weight, p.o., was administered to the control group (Cap). The third group was injected with 5% CMC-Na for six consecutive days along with 5 mg/kg of Cisplatin in physiological saline solution intraperitoneally (i.p.). The second group received Cap (10 mg/kg/d, p.o.) in 5% CMC-Na (5 mL/kg). Six days straight after receiving an injection of 5 mg/kg of Cisplatin intraperitoneally (i.p.), the fourth group was given Cap (10 mg/kg/d, p.o.) in 5% CMC-Na. Every group receives a cap or vehicle twice a day. Data from our preliminary experiments are used to determine the chosen Cap concentration and the dose administration schedule without causing any intestinal damage in rats. Cisplatin-Induced acute kidney injury (CI-AKI) Mouse Model [9] Researchers recapitulated a model of Cisplatin-induced acute kidney injury as previously described (Kim et al., 2012). Briefly, male 8–12-weeks C57BL/6 mice were deprived of food and water for 18 h prior to induction. Researchers used male mice only as female mice are more resistant to renal injury (Müller et al., 2002; Wei et al., 2005; Yang et al., 2010). Cisplatin was prepared for injection by dissolving at 1 mg/ml in sterile saline followed by a 30-min incubation in a 37°C water bath to ensure dissolution while protecting from light. Mice were then injected intraperitoneally with 25 mg/kg, at which time food and water were returned. Mice were sacrificed 72 h following cisplatin injection following a terminal retroorbital bleed. In addition to serum collection, kidneys were harvested and prepared for further study. Healthy control groups either underwent fasting and dehydration with no cisplatin injection or received food and water ad libitum. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
AFTER RAPID IV ADMIN /TO HUMAN PATIENTS/ ... THE DRUG HAS AN INITIAL ELIMINATION HALF-LIFE IN PLASMA OF 25-50 MIN; CONCNS OF TOTAL DRUG, BOUND & UNBOUND, FALL THEREAFTER, WITH A HALF-LIFE OF 24 HR OR LONGER. MORE THAN 90% OF THE PLATINUM IN THE BLOOD IS COVALENTLY BOUND TO PLASMA PROTEINS. HIGH CONCNS ... ARE FOUND IN THE KIDNEY, LIVER, INTESTINES, & TESTES, BUT THERE IS POOR PENETRATION INTO THE CNS. THE DRUG HAS A BIPHASIC PLASMA-DECAY CURVE WITH AN INITIAL HALF-LIFE OF 22 MIN (PROBABLY ELIMINATION) IN DOGS. HIGH TISSUE CONCNS HAVE BEEN FOUND IN KIDNEY, LIVER, OVARY, TESTIS, & UTERUS. AFTER IV INJECTION OF ANIMALS WITH CISPLATIN, PLASMA LEVELS DECLINE BIPHASICALLY. 24 HR URINARY EXCRETION OF PLATINUM IS EXTENSIVE WITH A FINAL URINARY RECOVERY OF 70-90%. PLATINUM IS INITIALLY DISTRIBUTED IN NEARLY ALL OF THE TISSUES, WITH THE HIGHEST LEVELS IN KIDNEY, LIVER, OVARY, UTERUS, SKIN & BONE, BUT THERE IS NO PREFERENTIAL UPTAKE OF PLATINUM BY TUMORS. AFTER IV ADMIN, MANY SPECIES (RAT, MOUSE, DOG) SHOW THE SAME GENERAL ORGAN DISTRIBUTION. ALL TISSUES TAKE UP PLATINUM, FOLLOWED WITHIN THE FIRST HR BY AN ACCUMULATION IN KIDNEY, LIVER, MUSCLE & SKIN. AFTER 24 HR, TISSUE:PLASMA DRUG RATIOS ARE GREATER THAN 1 IN OTHER TISSUES; THESE ARE MAINTAINED FOR AT LEAST A WK IN DOGS ... UP TO 4 WK AFTER A SINGLE DOSE, PLATINUM IS STILL DETECTABLE IN KIDNEY, LIVER, SKIN & LUNG. ... 18 HR AFTER IV INJECTION /OF RADIOACTIVE PLATINUM/ INTO RABBITS, KIDNEY & LIVER SHOWED THE HIGHEST LEVELS OF RADIOACTIVITY. For more Absorption, Distribution and Excretion (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (14 total), please visit the HSDB record page. Metabolism / Metabolites Cisplatin can react in a nonenzymatic manner with water in vivo to form monoaquo & diaquo species following dissociation of the chloride groups. These metabolites extensively bind to protein (>90%) & thus have minimal cytotoxicites but the non-protein bound, ultrafilterable reactive species are cytotoxic. Biological Half-Life AFTER RAPID IV ADMIN /TO HUMAN PATIENTS/ THE DRUG HAS AN INITIAL ELIMINATION HALF-LIFE IN PLASMA OF 25-50 MIN; CONCN DECLINE SUBSEQUENTLY WITH A HALF-LIFE OF 24 HR OR LONGER. THE DRUG HAS A BIPHASIC PLASMA-DECAY CURVE WITH AN INITIAL HALF-LIFE OF 22 MINUTES (PROBABLY ELIMINATION) IN DOGS. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Cisplatin is an antineoplastic cytostatic drug. Cisplain is deep yellow solid. Soluble in water, and in sodium chloride solution. Slowly changes from the cis to the trans form in aqueous solution. Soluble in dimethylformamide. Insoluble in most common solvents. Indications: Cisplatin is indicated for the following conditions: Single agent for the treatment of transitional cell bladder carcinoma that is no longer amenable to local treatment such as surgery and/or radiation therapy. Locally advanced or metastatic transitional cell carcinoma involving the renal pelvis, ureter, bladder and/or urethra. In combination with radiation treatment to treat bilharzial bladder cancer and together with doxorubicin and cyclophosphamide to treat locally advanced bladder cancer. The palliative treatment of recurrent or metastatic squamous cell carcinomas of the head or neck. Treatment of lung cancer, principally as a component of various chemotherapeutic regimens in the treatment of non-small cell lung carcinomas. It is often combined with other agents such as etoposide, vinblastine or vindesine to obtain a better response rate in lung cancer. Its use alone has some value but in combination the results are more noticeable in the palliative treatment of recurrent or advanced squamous cell carcinoma of the cervix and metastatic testicular carcinoma. Other types of carcinomas in which cisplatin has been tried included the following: osteogenic sarcoma, neuroblastoma and recurrent brain tumors in children, advanced esophageal carcinoma and advanced prostatic carcinoma. In combination with agents such as bleomycin, methotrexate, vincristine or vinblastine, fluorouracil in various regimes (all together or singularly depending on the protocol and the carcinoma type). Combinations of these agents have been reported to have a better response rate than if cisplatin were used alone. HUMAN EXPOSURE: Summary: Main Risks and Target Organs: The main risks experienced during cisplatin therapy and overdosage include nephrotoxicity, electrolyte disturbances, myelosuppression, neurotoxicity, anaphylactic reactions and ototoxicity. Nausea and vomiting can be severe. Rarer risks include cardiovascular effects, ocular effects, and hepatic effects. Most effects of overdosage are not usually seen immediately, but occur several days to months after the event. The causes of death from an overdose from cisplatin include myelosuppression, renal failure and tetany. Summary of Clinical Effects: Renal toxicity is cumulative and seen usually after several courses of cisplatin or after overdose. Disturbances in electrolytes can be a long term manifestation due to the cisplatin induced renal tubular dysfunction. Hypomagnesemia, hypocalcemia and hypokalemia are commonly seen in cisplatin induced renal toxicity and can persist for months after termination of therapy. Hematological effects of cisplatin (myelosuppression and anemia) are cumulative and in overdosage the hematopoietic system must be supported to prevent complications of infection. Cisplatin induces marked nausea and vomiting in almost all patients. Anaphylactoid reactions have occurred during normal therapy with cisplatin and must be treated vigorously. Cisplatin causes electrolyte disturbances which are a direct result of cisplatin induced renal tubular dysfunction. Cisplatin causes marked excretion of calcium, magnesium and potassium and to a lesser extent zinc, copper and amino acids. These disturbances must be corrected to prevent complications. Clinical features: Renal toxicity is manifested by an increase in serum creatinine, BUN, serum uric acid and/or a decrease in creatinine clearance and glomerular filtration rate. The renal impairment is a direct result of cisplatin induced renal tubular damage leading ultimately to renal failure. Disturbances have been seen in serum electrolytes due principally to cisplatin induced renal tubular dysfunction. Patients subsequently develop Hypomagnesemia, hypocalcemia and hypokalemia and to a lesser extent hypophosphatemia and hyponatremia. Cisplatin produces marked nausea and vomiting in almost all patients to the extent that some patients experience anticipatory nausea and vomiting. Diarrhea has also occurred but with less frequency than nausea and vomiting. Ototoxicity develops in various degrees on cisplatin therapy. In larger and prolonged dosing with cisplatin the ototoxicity can be irreversible. Myelosuppression is a common problem seen as leucopenia, thrombocytopenia and anemia and if severe enough can cause the death of the patient. Myelosuppression can be cumulative. Anaphylactoid reactions can occur when cisplatin is given. Cardiovascular effects are rare but include bradycardia, left bundle branch block and congestive heart failure. Hepatic enzyme activities in the sera become elevated including AST (SGOT) and ALT (SGPT). Precautions: Extreme care should be taken by persons preparing and administering cisplatin and those handling the urine of treated patients. Routes of entry: Cisplatin is not effective when administered orally. Dermal: Cisplatin is not administered dermally. Avoid dermal contact and absorption during administration. Eye: Eye contamination may be a possible source of poisoning during intravenous administration of cisplatin. Parenteral: Cisplatin is only available in the injectable form. The parenteral routes, intravenous, intra-arterial and intraperitoneal, have all been used in cisplatin therapy and poisoning would most likely occur by these three routes. Absorption by route of exposure: Intravenous: Totally absorbed after intravenous use. Rapid intravenous injection of cisplatin over 1 to 5 minutes or rapid intravenous infusion over 15 minutes or one hour, results in peak plasma concentrations immediately. When cisplatin is administered by intravenous infusion over 6 to 24 hours the plasma concentrations of total platinum increase gradually during the infusion and reach peak concentrations immediately following the end of the infusions. When mannitol is given at the same time as cisplatin, the peak plasma concentrations of non protein-bound platinum appears to be increased. Intra-arterial: When cisplatin is administered by intra-arterial infusion, the local tumor exposure of the drug is increased as compared with intravenous administration. Intraperitoneal: Cisplatin is rapidly and well absorbed systemically following intraperitoneal administration. This route gives 50 to 100% plasma concentration in comparison with the intravenous route. Intraperitoneal fluid concentration of the drug is greatly increased as compared with intravenous administration. Distribution by route of exposure: Following the intravenous administration of Cisplatin, the drug is widely distributed into body fluids and tissues. The highest concentrations can be seen in the kidneys, liver and intestines, and can persist for up to 2 to 4 weeks. However, concentrations can also be found in the muscles, bladder, testes, prostate, pancreas and spleen. Cisplatin has also been found in the following tissues; small and large intestines, adrenals, heart, lungs, lymph nodes, thyroid, gall bladder, thymus, cerebrum, cerebellum, ovaries and uterus. Platinum appears to accumulate in body tissues following administration of cisplatin and has been detected in many of these tissues for up to 6 months after the last dose of the drug. Platinum also has been found in leucocytes and erythrocytes. Cisplatin and any platinum-containing products are rapidly and extensively bound to tissue and plasma proteins, including albumin, gamma-globulins and transferrin. Binding to tissue and plasma proteins appears to be essentially irreversible with the bound platinum remaining in plasma during the lifespan of the albumin molecule. Protein binding increases with time and less than 2 to 10% of platinum in blood remains unbound several hours after intravenous administration of cisplatin. The extent of protein binding is about 90% and this occurs essentially within the first two hours after a dose. Penetration into the central nervous system (CNS) does not occur readily. The resultant levels are low in the CNS, but significant amounts of cisplatin can be detected in intracerebral tumor tissue and edematous brain tissue adjacent to the tumor. In healthy brain tissue concentrations appear to be low. Metabolism: The metabolic fate of cisplatin has not been completely elucidated. There is little evidence to date that the drug undergoes enzymatic biotransformation. The cisplatin molecule has chloride ligands on it and it is believed that these are displaced by water thus forming positively charged platinum complexes that react with nucleophilic sites. Their rate and extent depends on the strength, concentration and accessibility of the nucleophiles. The chemical identities of the metabolites of cisplatin have been found but have yet to be identified. There is a strong possibility that cisplatin and its metabolites undergo enterohepatic circulation. Elimination by route of exposure: Intact cisplatin and its metabolites are excreted principally in urine. It occurs predominantly via glomerular filtration but there is some evidence that secretion and reabsorption of cisplatin and its metabolites also occurs. Initially renal clearance of total platinum equals creatinine clearance and represents elimination of non-protein bound platinum molecules including intact cisplatin. As extensive protein binding occurs then clearance declines rapidly, resulting in a prolonged excretory phase. The ultimate rate of fall of total plasma platinum concentration is governed by the rate of degradation of plasma proteins bearing bound platinum. A small amount of cisplatin is excreted via the bile and saliva. Elimination half-life of cisplatin (Adults): Normal renal function: 2 to 72 hr. End stage renal disease: 1 to 240 hr. Mode of Action: Toxicodynamics: Cisplatin appears to be cycle-phase nonspecific and will cause cell death in all cells. It is in those cells which turn over rapidly (tumor cells, skin cells, gastrointestinal cells, bone marrow cells) that cell death will occur at a faster rate than other cells with a slower turnover rate (e.g. muscle cells). Cisplatin exerts its antineoplastic activity when it has the cis-configuration and without a charge on the molecule. The trans-configuration is inactive. Pharmacodynamics: Cisplatin complex moves through cell membranes in an unionized form and this is achieved in the relatively high chloride concentration in the plasma. Intracellularly the concentration of chloride ions is lower than in the plasma and the chloride ligands on the cisplatin complex are displaced by water. The result is the formation of positively charged platinum complexes that are toxic to cells. The cisplatin molecule binds to the DNA molecule at the guanine bases and thus inhibits DNA synthesis, protein and RNA synthesis (the latter two are inhibited to a lesser degree). The drug forms intrastrand and interstrand cross links in the DNA molecule and appears to correlate well with the cytotoxicity of the drug. The tumor cells amass an overburden of mutations which lead eventually to the cell's death. Cisplatin also has immunosuppressive, radiosensitizing and antimicrobial properties. The exact mechanism of action of cisplatin is not yet understood but the drug has biochemical properties similar to those of bifunctional alkylating agents. Human Data: Adults: The major toxicity caused during cisplatin treatment is dose related and cumulative. For example, renal tubular function impairment can occur during the second week of therapy and if higher doses or repeated courses of cisplatin are given then irreversible renal damage can occur. Teratogenicity: There is positive evidence of human fetal risk, so the benefits in pregnant women must be weighed against the risk. Interactions: Nephrotoxic drugs: Cisplatin produces cumulative nephrotoxicity that can be potentiated by nephrotoxic drugs (aminoglycosides, cephalosporins and amphoteracin). Aminoglycosides: Concurrent administration of aminoglycosides within 1-2 weeks of cisplatin therapy has been associated with an increased risk of nephrotoxicity and renal failure. Therefore aminoglycosides should be used with extreme care during treatment. Cisplatin ototoxicity is enhanced with the use of loop diuretics. ANIMAL STUDIES: Cisplatin is carcinogenic in animals. Mutagenicity: Cisplatin is mutagenic in bacterial cultures and produces chromosome aberrations in animal cells in tissue cultures. Interactions The RNA synthesis in vitro by Escherichia coli RNA polymerase were found to be highly sensitive to cis-platin inhibition. The degree of inhibition was in proportion to the length of time of template preincubation with cisplatin. It was found that adriamycin significantly enhanced the inhibitory effect of cisplatin & the total effect was greater than the sum of the effects of each drug used individually. A549 lung cancer cells were treated simultaneously with cisplatin (0, 1.25, 2.5, and 5 ug/ml) and other cytotoxic agents. Cisplatin additively incr the cytotoxic effects of etoposide, mitomycin C, adriamycin, 5-fluorouracil and 1-beta-D-arabinofuranosylcytosine, but antagonized those of vincristine, vindesine, vinblastine and podophyllotoxin. The antagonism between cisplatin and vincristine was also observed with HT29 colon cancer cells, NC65 renal carcinoma cells and A431 epidermoid carcinoma cells when these cells were simultaneously exposed to both agents. When A549 cells were exposed to cisplatin and vincristine sequentially (6 hr incubation with each agent), the antagonism between them was evident when the cells were pretreated with cisplatin, but not when treated in the opposite sequence. A study was conducted to investigate whether the antiemetic drug metoclopramide, a benzamide derivative (4-amino-N-2-(diethylaminoethyl)-5-chloro-2-methoxybenzamide), potentiates the effect of cis-platin on squamous cell carcinoma. Human squamous cell carcinoma of the head and neck (tumor line AB and EH) xenografted to nude mice were used. Two administration schedules were tested: (a) metoclopramide (2.0 mg/kg ip) given 1 hr before cis-platin (7.5 mg/kg ip); and (b) metoclopramide (3 x 2.0 mg/kg) given concomitant to, 24, and 48 hr after cis-platin (7.5 mg/kg) administration. Treatment efficacies were compared using the area under the growth curves, tumor vol, and specific growth delay. There was no mortality and no wt loss of significance in any treatment group. Metoclopramide alone did not induce any significant reduction in area under the growth curves tumor vol, or specific growth delay with either treatment schedule. Cis-platin alone gave a significant reduction of tumor growth in tumor line AB but not in tumor line EH. In schedule (a), the addition of metoclopramide did not give any additive effect. In schedule (b), for both tumor lines, metoclopramide enhanced the effect of cis-platin by significantly reducing the area under the growth curves (AB: p < 0.0001; EH: p < 0.001) and incr specific growth delay (AB: p < 0.012; EH: p < 0.001) when compared to the tumors given cis-platin alone. The ability of nifedipine, a dihydropyridine class calcium channel blocker, to overcome cis-platin resistance in a murine tumor line variant B16a-platinum, developed for resistance to cis-platin, was examined. Nifedipine significantly enhanced the antitumor actions of cis-platin against primary subcutaneous B16A-platinum tumors and their spontaneous pulmonary metastases. The pharmacokinetics and dose response interactions in vivo between nifedipine and cis-platin were also characterized. The in vivo efficacy of nifedipine and other calcium active compounds including structurally similar calcium channel blockers (nimodipine, nicardipine) from the dihydropyridine class, structurally different calcium channel blockers from the benzothiazepine (diltiazem) and the phenylalkylamine (verapamil) classes, and calmodulin antagonists (trifluoperazine and calmidazolium) were examined for their ability to enhance the antitumor action of cis-platin. Nifedipine was included as the standard or reference compound. All compounds studied failed to enhance the antitumor actions of cis-platin. For more Interactions (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (20 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral approx 20 mg/kg LD50 Rat oral 25,800 ug/kg LD50 Rat ip 6400 ug/kg LD50 Rat sc 8100 ug/kg For more Non-Human Toxicity Values (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (15 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Oncogene. 2003 Oct 20;22(47):7265-79. [2]. Biol Chem. 2000 Dec 15;275(50):39435-43. [3]. Cancer Res. 1988 Aug 15;48(16):4484-8. [4]. J Pharmacol Exp Ther. 2002 Jul;302(1):8-17. [5]. Am J Physiol. 1996 Apr;270(4 Pt 2):F700-8. [6]. Int J Cancer. 1992 Apr 22;51(1):108-15 [7]. Cancer Res. 2014 Jul 15;74(14):3913-22. |
| 其他信息 |
See also: Cisplatin (annotation moved to).
Mechanism of Action Cisplatin appears to enter cells by diffusion. The chloride atoms may be displaced directly by reaction with nucleophiles such as thiols; replacement of chloride by water yields a positively charged molecule & is probably responsible for formation of the activated species of the drug, which then reacts with nucleic acids & proteins. ... High concns of the anion stabilize the drug, explaining the effectiveness of chloride diuresis in preventing nephrotoxicity. ... The platinum complexes can react with DNA, forming both intrastrand & interstrand cross-links. The N(7) of guanine is very reactive, & platinum cross-links between adjacent guanines on the same DNA strand; guanine-adenine cross-links also readily form. The formation of interstrand crosslinks is a slower process & occurs to a lesser extent. DNA adducts formed by cisplatin inhibit DNA replication & transcription & lead to breaks & miscoding. The ability of patients to form & sustain DNA-platinum adducts in peripheral white blood cells has been correlated with response to treatment, indicating that pharmacogenetic factors or environmental exposures common to tumor & normal tissues may influence response. At present, there is no conclusive association between a single type of biochemical DNA adduct & cytotoxicity. The specificity of cisplatin with regard to phase of the cell cycle appears to differ among cell types, although the effects on cross-linking are most pronounced during the S phase. A poorly differentiated squamous cell carcinoma of the head & neck heterotransplanted to nude mice was used for analyses of chemotherapeutically induced cell cycle perturbations. The tumor in its later passages in nude mice, was treated with cis-platin. There was an initial incr of the fraction of cells in the S phase, concomitant with a redn of the fraction of cells in G0 + G1 phase. When these perturbations were normalized a transient incr of the fraction of cells in G2 + M phase was observed. Cisplatin caused an initial transient depression of DNA synthesis. The cytokinetic response of 3 murine (AC) and human (GB-1 and GB-2) glioma cell lines to cis-platin was investigated by flow cytometry. Using the 5-bromodeoxyuridine-Hoechst technique, percentages of cultured glioma cells in the various phases of the cell cycle, and relative phase duration were calculated. In the presence of cis-platin IC10 (a concentration in which 10% inhibition of cell growth is induced as compared to controls), perturbations of the cell cycle in murine and GB-1 cells included G2 delay or block, decr transit velocity from G1 to S phase, and prolongation of G1 phase. The mean cell cycle time incr 1.4 times in murine and 1.6 times in GB-1 as compared to controls. In cis-platin IC50-treated GB-2 cells, the mean cell cycle time was prolonged 3 times longer than control; however, duration of each phase could not be calculated because of significant perturbation of cell cycle. Studies have been carried out of the inhibition of ribonucleotide reductase (EC 1.17.4.1) purified from Escherichia coli by cis-platin. Under anaerobic conditions, using the dithiol reduced form of the enzyme, it was found that ribonucleotide reductase is extremely sensitive to cis-platin; > 90% inhibition was achieved with 2 fold molar excess of platinum reagent even at 10-8 M enzyme. Inhibition was essentially instantaneous and irreversible to G-25 gel filtration. The site of inhibition was found to be the B1 subunit. Transplatin was much less effective. Inhibition of the enzyme by cis-platin (molar ratio cis-platin:B1 = 4.3) led to a decr in thiol titre corresponding to approx 1 thiol group per dimer of B1 subunits under conditions leading to 94% inactivation of the ribonucleotide reductase activity. For more Mechanism of Action (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (10 total), please visit the HSDB record page. Therapeutic Uses Antineoplastic Agents; Cross-Linking Reagents; Radiation-Sensitizing Agents CISPLATIN IS USED IN HUMAN MEDICINE FOR THE TREATMENT OF A VARIETY OF MALIGNANCIES ... TESTICULAR TUMORS, MALIGNANT MELANOMA, OSTEOGENIC SARCOMA & CARCINOMAS OF THE BLADDER, LUNG (NON-SMALL-CELL), UTERINE CERVIX, OVARY & SQUAMOUS CARCINOMA OF THE HEAD & NECK REGION. THE USUAL IV DOSE OF CISPLATIN IS 20 MG/SQ M/DAY FOR 5 DAYS OR 100 MG/SQ M, GIVEN ONCE EVERY 4 WK. DOSES AS HIGH AS 40 MG/SQ M DAILY FOR 5 DAYS HAVE BEEN USED ALONE OR TOGETHER WITH CYCLOPHOSPHAMIDE FOR THE TREATMENT OF PATIENTS WITH ADVANCED OVARIAN CANCER, BUT RESULT IN GREATER RENAL, HEARING, & NEUROLOGICAL TOXICITY. TO PREVENT RENAL TOXICITY, HYDRATION OF THE PATIENT BY THE INFUSION OF 1-2 L OF NORMAL SALINE PRIOR TO TREATMENT IS RECOMMENDED. THE APPROPRIATE AMT OF CISPLATIN IS THEN DILUTED IN A SOLN OF DEXTROSE & SALINE & ADMIN IV OVER A PERIOD OF 6-8 HR. SINCE ALUMINUM REACTS WITH AND INACTIVATES CISPLATIN, IT IS IMPORTANT NOT TO USE NEEDLES OR OTHER EQUIPMENT THAT CONTAINS ALUMINUM WHEN PREPARING OR ADMINISTERING THE DRUG. From June 1977 to June 1987, 68 patients were treated with cisplatin for recurrent squamous cell carcinoma of the cervix as the primary chemotherapeutic agent & evaluated for response &/or survival. Patients were treated with 50-100 mg/sq m of cisplatin at 3 wk intervals. Patients with disease confined to the chest had a 53% complete response rate with an overall response rate of 73%. Patients with localized pelvic recurrence or persistence demonstrated no complete responses & a 21% overall response rate. Isolated chest metastases responded more frequently to cisplatin than pelvic recurrences (73% vs 22%, p=0.0007); however, location of recurrence did not significantly alter survival (mean 22.7 mo vs 14.1 mo; p=0.24). Concomitant disease in other locations reduced the likelihood of response in the chest (p>0.05) by virtue of lack of response in those other sites. Lesion size, clinical stage, patient age, & duration from primary treatment to recurrence were not of significance with regard to response or survival. For more Therapeutic Uses (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (6 total), please visit the HSDB record page. Drug Warnings THE DRUG SHOULD NOT BE ADMINISTERED THROUGH AN ALUMINUM NEEDLE, SINCE ALUMINUM REACTS WITH AND INACTIVATES THE DRUG. Possible inactivation of cis-platinum may occur when sodium bisulfite is added to cis-platinum in iv fluid prior to admin. A Mallory-Weiss tear was reported in a patient as a complication of cancer chemotherapy including cis-platin. It is suggested that the Mallory-Weiss syndrome should be included in the differential diagnosis of any patient with epigastric pain, hematemesis, or melena after chemotherapy induced retching or vomiting. The auditory function of subjects receiving cisplatin for genitourinary tumors & head & neck cancers was serially monitored with conventional audiometry & with a high frequency testing system. Results reveal a high incidence of nonreversible cochlear toxicity with a predilection for involvement of the higher frequencies. Cochlear toxicity was detected earlier with the high frequency evaluation system. For more Drug Warnings (Complete) data for CIS-DIAMINEDICHLOROPLATINUM (21 total), please visit the HSDB record page. |
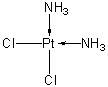
| 分子式 |
CL2H6N2PT
|
|
|---|---|---|
| 分子量 |
300.05
|
|
| 精确质量 |
296.939
|
|
| 元素分析 |
Cl, 23.63; H, 2.02; N, 9.34; Pt, 65.02
|
|
| CAS号 |
15663-27-1
|
|
| 相关CAS号 |
|
|
| PubChem CID |
2767
|
|
| 外观&性状 |
Yellow solid powder
|
|
| 密度 |
3.7
|
|
| 熔点 |
270ºC
|
|
| LogP |
1.595
|
|
| tPSA |
52.04
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
5
|
|
| 分子复杂度/Complexity |
7.6
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
[Cl-][Pt]([NH3+])([NH3+])[Cl-]
|
|
| InChi Key |
LXZZYRPGZAFOLE-UHFFFAOYSA-L
|
|
| InChi Code |
InChI=1S/2ClH.2H3N.Pt/h2*1H;2*1H3;/q;;;;+2/p-2
|
|
| 化学名 |
(SP-4-2)-diamminedichloroplatinum; platinum, diaminedichloro-, cis-
|
|
| 别名 |
Cismaplat; Cisplatina; cisplatinous diamine dichloride; cisplatinum; cisplatinum II; cisplatinum II diamine dichloride; CDDP; DDP; cisDDP; cisdiamminedichloro platinum (II); cisdiamminedichloroplatinum; Cisdichloroammine Platinum (II); CPDD; Cysplatyna; DDP; PDD; Peyrones Chloride; Peyrones Salt; CACP; Platinoxan; platinum diamminodichloride. Trade names (US): Platinol; PlatinolAQ. Trade names (other countries): Abiplatin; Blastolem; Briplatin; Cisplatyl; Citoplatino; Citosin; Lederplatin; Metaplatin; Neoplatin; Placis; Platamine; Platiblastin; PlatiblastinS; Platinex; Platinol AQ; PlatinolAQ VHA Plus; Platiran; Platistin; Platosin.
|
|
| HS Tariff Code |
2843.90.0000
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
Note: Cisplatin一般不用DMSO溶解,因为铂类药物在DMSO中易失活!另外, Cisplatin在溶液中不稳定,请现配现用!
配方 1 中的溶解度: 10 mg/mL (33.33 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 20 mg/mL (66.66 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More配方 3 中的溶解度: Saline: 3 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3328 mL | 16.6639 mL | 33.3278 mL | |
| 5 mM | 0.6666 mL | 3.3328 mL | 6.6656 mL | |
| 10 mM | 0.3333 mL | 1.6664 mL | 3.3328 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03558087 | Active Recruiting |
Drug: Nivolumab Drug: Cisplatin |
Bladder Cancer | Matthew Galsky | July 13, 2018 | Phase 2 |
| NCT01670500 | Active Recruiting |
Drug: Cisplatin Drug: Doxorubicin |
Breast Cancer | Beth Israel Deaconess Medical Center |
October 2012 | Phase 2 |
| NCT03809637 | Active Recruiting |
Drug: Pemetrexed, cisplatin | Yonsei University | Sarcoma | January 10, 2017 | Phase 2 |
| NCT03345784 | Active Recruiting |
Drug: Cisplatin Drug: Adavosertib |
Cervical Carcinoma Vaginal Carcinoma |
National Cancer Institute (NCI) |
May 29, 2018 | Phase 1 |
| NCT04003636 | Active Recruiting |
Drug: Cisplatin Drug: Placebo |
Biliary Tract Carcinoma | Merck Sharp & Dohme LLC | September 24, 2019 | Phase 3 |
The inhibition of tumor growth was enhanced by HemoHIM administration in melanoma-bearing mice which were injected with cisplatin.BMC Cancer.2009 Mar 17;9:85. |
|---|
Growth inhibition effect of cisplatin and HemoHIM on melanoma cellsin vitro.BMC Cancer.2009 Mar 17;9:85. |
HemoHIM administration promotes immune responses for tumor surveillance in melanoma-bearing mice which were injected with cisplatin.BMC Cancer.2009 Mar 17;9:85. |