| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
CYP (cytochrome P450); antifungal
|
|---|---|
| 体外研究 (In Vitro) |
克霉唑(商品名 Canesten 和 Lotrimin)是一种抗真菌药物,用于治疗人类和动物的真菌疾病,包括阴道酵母菌感染、鹅口疮和癣。它还用于缓解脚气和股癣。它经常以多种剂型在柜台出售,包括乳膏和组合药物。它也有锭剂或锭剂的形式(需要处方)。耳部感染通常用滴耳剂等液体治疗。克霉唑是一种抗真菌药物,其影响胃 H,K-ATP 酶的作用与 Na,K-ATP 酶的作用相同。由于其高疏水性,克霉唑与膜非极性核心的膜域处的离子泵相互作用。 5.2 microM 的半饱和浓度会降低酶活性。使用电致变色苯乙烯基染料 RH421 研究了各种泵循环部分反应,该染料已广泛用于检查 P 型 ATP 酶转运机制。
|
| 体内研究 (In Vivo) |
在治疗阴道念珠菌病方面,克霉唑阴道片的治愈率与常规制霉菌素阴道片相当。目前还没有发表与制霉菌素阴道乳膏或发泡阴道片剂的比较——一些临床医生首选制霉菌素剂型。库霉唑在对制霉菌素和两性霉素B等其他抗真菌药物没有反应的患者中也取得了成功。滴虫性阴道炎的结果并不令人印象深刻。局部应用克霉唑可有效治疗念珠菌或皮肤癣菌引起的皮肤感染。在比较试验中,克霉唑乳膏在治疗皮肤真菌病方面与惠特菲尔德软膏和托萘酯一样有效,在治疗皮肤念珠菌病方面与制霉菌素一样有效。克霉唑外用制剂通常耐受性良好,但在少数情况下,局部刺激需要停止治疗。口服克霉唑治疗已治愈念珠菌败血症和泌尿系和肺部念珠菌病。其他类型的严重真菌感染(包括肺曲霉病)的结果令人失望。口服克霉唑治疗的一个限制因素是胃肠道紊乱和神经反应的高发率[1]。
|
| 酶活实验 |
克霉唑2是一种合成咪唑衍生物,主要用于局部治疗由酵母菌和皮肤癣菌引起的阴道和皮肤感染。在体外,它对念珠菌属、毛癣菌属、小孢子菌属和圆形糠秕孢子虫最为有效。此外,它对某些革兰氏阳性细菌具有一定的体外活性,在非常高的浓度下对毛滴虫具有活性。[1]
抗真菌药物克霉唑以类似于观察到的Na,K-ATP酶的方式抑制胃H,K-ATPase的功能。由于该化合物的高疏水性,克霉唑和离子泵之间的相互作用发生在膜非极性核心的膜域。酶活性被5.2微M的半饱和浓度抑制。用电致变色苯乙烯基染料RH421分析了泵循环的各种部分反应,RH421已被广泛用于研究P型ATP酶的转运机制。我们发现,克霉唑与H,K-ATP酶的相互作用在泵的E(1)状态下引入了一个添加到Post Albers方案中的“死端”分支。在这种抑制状态下,离子结合位点对质子的亲和力显著增强,即使在pH 8.5下也能结合多达两个质子。泵的抑制可以通过降低pH值或增加K(+)浓度来逆转。允许解释所有实验的机制建议与发表的Na,K-ATPase类似[2]。 |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Because clotrimazole is generally not significantly absorbed, drug interactions are not a major issue with its use. Mainly hepatic. The topical form is minimally absorbed in the serum and tissues. Clotrimazole is a lipophilic drug, and has been shown to be secreted in breastmilk in animal studies. There are limited data available regarding the volume of distribution following oral troche administration. GIVEN ORALLY OR IV WAS ABSORBED, DISTRIBUTED, ELIMINATED READILY. EXCRETED AS INACTIVE METABOLITE IN BILE, LITTLE IN URINE. Absorption of clotrimazol is less than 0.5% after application to the intact skin: from the vagina, it is 3 to 10%. Fungicidal concentrations remain in the vagina for as long as 3 days after application of the drug. The small amount absorbed is metabolized in the liver and excreted in bile. In adults, an oral dose of 200 mg per day will give rise to plasma concentrations of 0.2 to 0.35 ug/ml. Only very small amounts of clotrimazole appear to be absorbed systemically following topical application to the skin. Following application to the skin, highest concentrations of clotrimazole are present in the stratum corneum; lower drug concentrations occur in the stratum spinosum and the papillary and reticular dermis. Small amounts of clotrimazole are absorbed systemically when the drug is administered intravaginally. Following intravaginal administration of radiolabeled clotrimazole in patients with normal or inflamed vaginal mucosa, peak serum concentrations of clotrimazole 24 hours after insertion of a single 100 mg tablet of the drug are 0.03 ug/ml and peak serum concentrations 24 hours after administration of a cream containing 50 mg of the drug are 0.01 ug/ml. About 3-10% of an intravaginal dose of the drug reaches systemic circulation, principally as metabolites. Clotrimazole is absorbed from the gastrointestinal tract ... and excreted in the feces and urine. When applied topically clotrimazole penetrates the epidermis but there is little if any systemic absorption. Slight absorption has been reported following the administration of vaginal tablets. Metabolism / Metabolites Hepatic (metabolized to inactive metabolites). Clotrimazole ... is metabolized in the liver to inactive compounds ... . The effect of the antifungal imidazole compound, clotrimazole, on the metabolism of benzo[a]pyrene was studied in cultured keratinocytes prepared from BALB/c mouse epidermis. Varying concentrations of clotrimazole added to the cultured keratinocytes resulted in a dose dependent inhibition of the activities of the microsomal cytochrome p450 dependent monooxygenases aryl hydrocarbon hydroxylase and 7-ethoxycoumarin O-deethylase. The major organic solvent soluble metabolites of benzo(a)pyrene identified in the cultured cells were trans-7,8-dihydro-7,8-dihydroxybenzo(a)pyrene, 9-hydroxybenzo(a)pyrene, and 3-hydroxybenzo(a)pyrene, although small amounts of trans-4,5-dihydro-4,5-dihydroxybenzo(a)pyrene, benzo(a)pyrene-quinones, and trans-9,10-dihydroxybenzo(a)pyrene were also present. The major organic solvent extractable metabolites of benzo(a)pyrene found in the extracellular culture medium were primarily the diols with smaller quantities of phenols and quinones. The major water soluble metabolites of benzo(a)pyrene present both intracellularly and extracellularly were glucuronide conjugates of 3-hydroxybenzo(a)pyrene, 9-hydroxybenzo(a)pyrene, and benzo(a)pyrene-3,6-dione and to a lesser extent sulfate conjugates (primarily of the trans-7,8-dihydro-7,8- dihydroxybenzo(a)pyrene). Clotrimazole inhibited the generation of organic solvent soluble and water soluble conjugates in a dose dependent manner. The in vitro metabolism of benzo(a)pyrene by microsomes prepared from control and benz(a)anthracene induced cultured keratinocytes was also inhibited by clotrimazole with greater inhibitory effect on benz(a)anthracene induced keratinocytes especially with respect to the formation of diols and quinones. The enzyme mediated covalent binding of benzo(a)pyrene to mouse keratinocyte DNA and protein was also substantially diminished by clotrimazole in a dose dependent fashion. These results indicate that clotrimazole, a widely used drug for the management of a variety of superficial dermatophyte infections of the skin, is a potent inhibitor of cytochrome p450 dependent transformation of polycyclic aromatic hydrocarbons in cultured murine keratinocytes. This system offers a convenient approach for studies as inhibitors of carcinogen metabolism in the epidermis. Hepatic (metabolized to inactive metabolites) Half Life: 2 hours Biological Half-Life Clotrimazole was given by mouth in a dose of 1.5 g to 7 healthy subjects and 47 patients and peak blood concentrations of up to 1 ug/ml were detected microbiologically at 2 or 4 hours. The half-life was between 3.5 and 5.5 hours. Clotrimazole was absorbed from the gastrointestinal tract and had a biological half-life of about 4 hours. Liver and kidney dysfunction had little influence on serum concentrations or half-life. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Clotrimazole interacts with yeast 14-α demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the membrane. In this way, clotrimazole inhibits ergosterol synthesis, resulting in increased cellular permeability. Clotrimazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms and the uptake of purine, impair triglyceride and/or phospholipid biosynthesis, and inhibit the movement of calcium and potassium ions across the cell membrane by blocking the ion transport pathway known as the Gardos channel. Interactions A synergistic effect of clotrimazole and certain anionic surfactants against a strain of Candida albicans was confirmed. Measurement of apparent partition coefficients indicated that lipophilic ion pairs between clotrimazole and anionic surfactants were formed. It is suggested that the synergistic effect of the drugs may be due to ion pair formation. Non-Human Toxicity Values LD50 Rat, male oral 708 mg/kg LD50 Mouse, male oral 923 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anti-Infective Agents, Local; Antifungal Agents; Growth Inhibitors CLOTRIMAZOLE IS A CHLORINATED IMIDAZOLE DERIVATIVE THAT IS USED TO TREAT TOPICAL FUNGAL, DERMATOPHYTE, & YEAST INFECTIONS. WHILE CLOTRIMAZOLE HAS MARKED IN VITRO ACTIVITY AGAINST MANY FUNGI, IT IS OF LITTLE VALUE IN TREATMENT OF SYSTEMIC MYCOSES. VAGINAL: 1 TABLET (100 MG) IS INSERTED DAILY FOR 1 WK FOR CANDIDAL VAGINITIS. TOPICAL: SUFFICIENT CREAM OR SOLN IS APPLIED TWICE DAILY TO SKIN INFECTED WITH CANDIDA ALBICANS, TRICOPHYTON, OR MICROSPORUM SPECIES. 2 WK OF THERAPY IS USUALLY SUFFICIENT. CLOTRIMAZOLE HAS BEEN USED INVESTIGATIONALLY FOR ORAL TREATMENT OF MUCOCUTANEOUS CANDIDIASIS. For more Therapeutic Uses (Complete) data for CLOTRIMAZOLE (12 total), please visit the HSDB record page. Drug Warnings PREPN OF CLOTRIMAZOLE ARE NOT INTENDED FOR OPHTHALMIC USE & SHOULD BE USED WITH CAUTION AROUND EYES. Clotrimazole lozenges should not be used for the treatment of systemic myotic infections. Clotrimazole Vaginal tablets ... single-dose therapy is not recommended for the treatment of severe vulvovaginal candidiasis. To achieve maximum theraputic effect of clotrimazole when the drug is administered orally as a lozenge, the lozenge must be dissolved slowly in the mouth. Therefore, patients receiving clotrimazole lozenges must be of such age and physical and/or mental condition that they can comprehend and follow administration instruction. Liver function tests should be conducted periodically during oral therapy with clotrimazole lozenges, especially in patients with preexisting hepatic impairment. Clotrimazole topical cream, lotion, and solution should be used during the first trimester of pregnancy only when the drug is considered essential to the welfare of the patient. Since it is not known whether clotrimazole is distributed into milk, the drug should be used with caution in nursing women. Pharmacodynamics Clotrimazole is a broad-spectrum antifungal agent that inhibits the growth of pathogenic yeasts by changing the permeability of cell membranes. The action of clotrimazole is fungistatic at concentrations of drug up to 20 mcg/mL and may be fungicidal _in vitro_ against Candida albicans and other species of the genus Candida at higher concentrations. Unfortunately, resistance to clotrimazole, which was rare in the past, is now common in various patient populations. Clotrimazole is generally considered to be a fungistatic, and not a fungicidal drug, although this contrast is not absolute, as clotrimazole shows fungicidal properties at higher concentrations. |
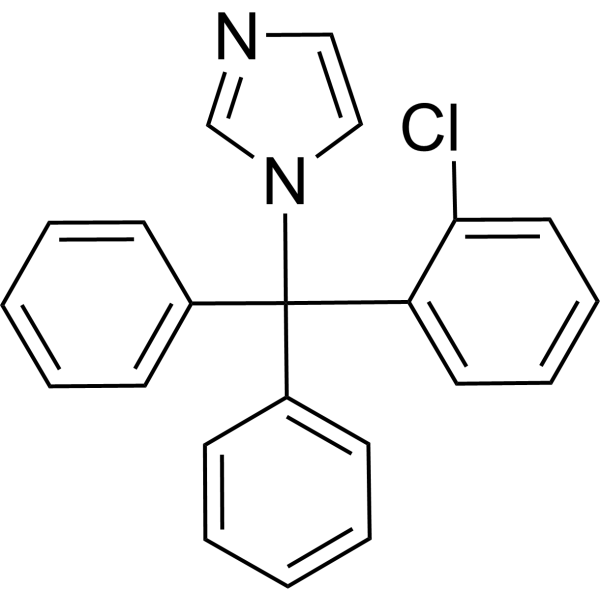
| 分子式 |
C22H17CLN2
|
|---|---|
| 分子量 |
344.84
|
| 精确质量 |
344.108
|
| 元素分析 |
C, 76.63; H, 4.97; Cl, 10.28; N, 8.12
|
| CAS号 |
23593-75-1
|
| 相关CAS号 |
Clotrimazole-d5;1185076-41-8
|
| PubChem CID |
2812
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
482.3±40.0 °C at 760 mmHg
|
| 熔点 |
147-149ºC
|
| 闪点 |
245.5±27.3 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.617
|
| LogP |
5.44
|
| tPSA |
17.82
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
396
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
VNFPBHJOKIVQEB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H
|
| 化学名 |
1-[(2-chlorophenyl)-diphenylmethyl]imidazole
|
| 别名 |
Canesten; Lotrimin; 23593-75-1; Lotrimin; Canesten; Mycelex; Mycosporin; Clotrimazol; Empecid; Clotrimazole
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~144.99 mM)
H2O : ~0.1 mg/mL (~0.29 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.25 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.25 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8999 mL | 14.4995 mL | 28.9990 mL | |
| 5 mM | 0.5800 mL | 2.8999 mL | 5.7998 mL | |
| 10 mM | 0.2900 mL | 1.4499 mL | 2.8999 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。