| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 靶点 |
DNA synthesis ( IC50 = 16 nM )
|
|---|---|
| 体外研究 (In Vitro) |
阿糖胞苷通过脱氧胞苷激酶 (dCK) 的作用磷酸化为三磷酸形式 (Ara-CTP),从而抑制 DNA 和 RNA 聚合酶的功能并与 dCTP 竞争掺入 DNA。阿糖胞苷的 IC50 为 16 nM,对野生型 CCRF-CEM 细胞的生长抑制活性高于对其他急性髓性白血病 (AML) 细胞的生长抑制活性[1]。 10 μM 的阿糖胞苷似乎会导致大鼠交感神经元凋亡;毒性最高的是100 μM,通过激活caspase-3和释放线粒体细胞色素-c,在84小时内杀死超过80%的神经元。 bax 缺失可延迟毒性,p53 敲低可减弱毒性[2]。
|
| 体内研究 (In Vivo) |
Cytarabine (250 mg/kg) 还会导致妊娠 Slc:Wistar 大鼠胎盘生长迟缓并增加胎盘迷路区胎盘滋养层细胞的凋亡。这个过程在治疗后三小时开始,在六小时达到峰值,并在四十八小时恢复到控制水平。值得注意的是,p53 蛋白和 p53 转录靶基因,包括 p21、cyclin G1、fas 和 caspase-3 活性显着增加[3]。使用较高剂量的阿糖胞苷似乎并不能提高其在人类中的抗白血病功效。阿糖胞苷对急性白血病非常有效,急性白血病会导致阿糖胞苷 G1/S 阻断和同步化,并以弱剂量相关的方式延长白血病 Brown挪威大鼠的生存时间[4]。
|
| 动物实验 |
Intraperitoneal (i.p.) injection of 250 mg/kg Cytarabine is administered to pregnant rats on Day 13 of gestation (GD13). Congenital defects and growth retardation are frequently found in perinatal fetuses under the circumstances of this experiment, but the incidence of fetal death is not noticeably elevated. Following treatment, six dams are killed by heart puncture under ether anesthesia at 1, 3, 6, 9, 12, 24, and 48 hours. The placentas are then collected. In GD13, six pregnant rats serve as controls. They receive an intraperitoneal injection of the same volume of PBS and are killed concurrently with the groups treated with cytarabine. Three of the six dams collected at each time point are utilized for histopathological examinations, and the remaining three are used for RT-PCR (reverse transcription-polymerase chain reaction) analysis.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Less than 20% of the orally administered dose is absorbed from the gastrointestinal tract. The primary route of elimination of cytarabine is metabolism to the inactive compound ara-U, followed by urinary excretion of ara-U. Less than 20% of a dose of conventional cytarabine is absorbed from the GI tract, and the drug is not effective when administered orally. Following subcutaneously or im injection of conventional cytarabine H 3, peak plasma concentrations of radioactivity occur within 20-60 min and are considerably lower than those attained after iv administration. Continuous iv infusions of conventional cytarabine produce relatively constant plasma concn of the drug in 8-24 hr. Cytarabine is rapidly and widely distributed into tissues and fluids, including liver, plasma, and peripheral granulocytes. Following rapid IV injection of cytarabine in one study, approximately 13% of the drug was bound to plasma proteins. Cytarabine crosses the blood-brain barrier to a limited extent. During a continuous IV or subcutaneous infusion, cytarabine concentrations in the CSF are higher than those attained after rapid IV injection and are about 40-60% of plasma concentrations. Most of an intrathecal dose of cytarabine diffuses into the systemic circulation but is rapidly metabolized and usually only low plasma concentrations of unchanged drug occur. The drug apparently crosses the placenta. It is not known if cytarabine or ara-U is distributed into milk. For more Absorption, Distribution and Excretion (Complete) data for CYTARABINE (7 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Cytarabine is rapidly and extensively metabolized mainly in the liver but also in kidneys, GI mucosa, granulocytes, and to a lesser extent in other tissues by the enzyme cytidine deaminase, producing the inactive metabolite 1-ß-d-arabinofuranosyluracil (ara-U, uracil arabinoside). After the initial distribution phase, more than 80% of the drug in plasma is present as ara-U. In the CSF, only minimal amounts of cytarabine are converted to ara-U because of low CSF concentrations of cytidine deaminase. Intracellularly, cytarabine is metabolized by deoxycytidine kinase and other nucleotide kinases to cytarabine triphosphate, the active metabolite of the drug. Cytarabine triphosphate is inactivated by a pyrimidine nucleoside deaminase, which produces the uracil derivative. The primary route of elimination of cytarabine is metabolism to the inactive compound ara-U (1-(beta)-D-arabinofuranosyluracil or uracilarabinoside), followed by urinary excretion of ara-U. In contrast to systemically administered cytarabine, which is rapidly metabolized to ara-U, conversion to ara-U in the CSF is negligible after intrathecal administration because of the significantly lower cytidine deaminase activity in the CNS tissues and CSF. The CSF clearance rate of cytarabine is similar to the CSF bulk flow rate of 0.24 mL/min. /Cytarabine liposome injection/ Cytarabine must be converted to the 5'-monophosphate nucleotide by deoxycytidine kinase to be active. Ara-cytidine diphosphate &/or ara-cytidine triphosphate are presumably the form that inhibit DNA polymerase & block ribonucleoside diphosphate reductase. Hepatic. Biological Half-Life 10 minutes After rapid IV injection of cytarabine, plasma drug concentrations appear to decline in a biphasic manner with a half-life of about 10 minutes in the initial phase and about 1-3 hours in the terminal phase. Cytarabine reportedly undergoes triphasic elimination in some patients. After intrathecal injection, cytarabine concentrations in the CSF reportedly decline with a half-life of about 2 hours. Peak levels were followed by a biphasic elimination profile with a terminal phase half-life of 100 to 263 hours over a dose range of 12.5 mg to 75 mg. In contrast, intrathecal administration of 30 mg of free cytarabine showed a biphasic CSF concentration profile with a terminal phase half-life of 3.4 hours. /Cytarabine liposome injection/ After iv admin, there is a rapid phase of disappearance of AraC (half-life = 10 min), followed by a slower phase of elimination with a half-time of about 2.5 hr ... After intrathecal admin of the drug at a dose of 50 mg/sq m ... peak concn of 1 to 2 mM are achieved, which decline slowly with a terminal half-life of approx 3.4 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Serum aminotransferase elevations occur in 5% to 10% of patients on conventional doses of cytarabine and a greater proportion (9% to 75%) at higher doses. However, the serum enzyme elevations are rarely associated with symptoms and are generally self-limited and resolve rapidly, rarely requiring dose modification. Cases of clinically apparent liver injury attributed to cytarabine have been reported but are uncommon. The time to onset was usually within the first few cycles of therapy, and the pattern of serum enzyme elevations ranged from cholestatic to hepatocellular. Immunoallergic and autoimmune features were generally not present. Antineoplastic regimens, including cytarabine, have been implicated in cases of sinusoidal obstruction syndrome and peliosis, but the role of cytarabine in these reactions was unclear. Many examples of liver injury attributed to cytarabine in the literature were typical of jaundice of sepsis rather than acute hepatocellular or cholestatic injury, although high doses of cytarabine may cause hyperbilirubinemia independent of hepatic injury. Likelihood score: C (probable cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the excretion of cytarabine into breastmilk. However, the drug has a short half-life of 2 to 3 hours after intravenous administration, so it should be eliminated from milk a day after intravenous administration. Very little information is available on the use of cytarabine during breastfeeding. In one case, a mother began breastfeeding her infant 3 weeks after receiving cytarabine, mitoxantrone and etoposide intravenously, with no apparent harm to her infant. After intrathecal administration of the liposomal formulation of cytarabine, drugs levels in plasma are barely detectable, and are unlikely to appear in milk in clinically relevant amounts. ◉ Effects in Breastfed Infants One mother received 3 daily doses of 6 mg/sq. m. of mitoxantrone intravenously along with 5 daily doses of etoposide 80 mg/sq. m. and cytarabine 170 mg/sq. m. intravenously. She resumed breastfeeding her infant 3 weeks after the third dose of mitoxantrone at a time when mitoxantrone was still detectable in milk. The infant had no apparent abnormalities at 16 months of age. However, after 3 weeks of abstinence from breastfeeding, it is unlikely that cytarabine was present in milk during breastfeeding. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 13% |
| 参考文献 |
|
| 其他信息 |
Cytarabine can cause developmental toxicity according to an independent committee of scientific and health experts.
Cytarabine appears as colorless crystals. Used as an antiviral agent. Crystals (from aqueous ethanol) or fluffy white powder. (NTP, 1992) Cytarabine is a pyrimidine nucleoside in which cytosine is attached to D-arabinofuranose via a beta-N(1)-glycosidic bond. Used mainly in the treatment of leukaemia, especially acute non-lymphoblastic leukaemia, cytarabine is an antimetabolite antineoplastic agent that inhibits the synthesis of DNA. It also has antiviral and immunosuppressant properties. It has a role as an antineoplastic agent, an antimetabolite, an antiviral agent and an immunosuppressive agent. It is a beta-D-arabinoside, a pyrimidine nucleoside and a monosaccharide derivative. It is functionally related to a cytosine. A pyrimidine nucleoside analog that is used mainly in the treatment of leukemia, especially acute non-lymphoblastic leukemia. Cytarabine is an antimetabolite antineoplastic agent that inhibits the synthesis of DNA. Its actions are specific for the S phase of the cell cycle. It also has antiviral and immunosuppressant properties. (From Martindale, The Extra Pharmacopoeia, 30th ed, p472) Cytarabine is a Nucleoside Metabolic Inhibitor. The mechanism of action of cytarabine is as a Nucleic Acid Synthesis Inhibitor. Cytarabine is a cytosine analogue and antineoplastic agent used largely in the therapy of acute leukemia. Cytarabine is associated with a low rate of transient serum enzyme and bilirubin elevations during therapy, but has only rarely been implicated in cases of clinically apparent acute liver injury with jaundice. Cytarabine is an antimetabolite analogue of cytidine with a modified sugar moiety (arabinose instead of ribose). Cytarabine is converted to the triphosphate form within the cell and then competes with cytidine for incorporation into DNA. Because the arabinose sugar sterically hinders the rotation of the molecule within DNA, DNA replication ceases, specifically during the S phase of the cell cycle. This agent also inhibits DNA polymerase, resulting in a decrease in DNA replication and repair. (NCI04) A pyrimidine nucleoside analog that is used mainly in the treatment of leukemia, especially acute non-lymphoblastic leukemia. Cytarabine is an antimetabolite antineoplastic agent that inhibits the synthesis of DNA. Its actions are specific for the S phase of the cell cycle. It also has antiviral and immunosuppressant properties. (From Martindale, The Extra Pharmacopoeia, 30th ed, p472) A pyrimidine nucleoside analog that is used mainly in the treatment of leukemia, especially acute non-lymphoblastic leukemia. Cytarabine is an antimetabolite antineoplastic agent that inhibits the synthesis of DNA. Its actions are specific for the S phase of the cell cycle. It also has antiviral and immunosuppressant properties. (From Martindale, The Extra Pharmacopoeia, 30th ed, p472) See also: Cytarabine; daunorubicin (component of). Drug Indication For the treatment of acute non-lymphocytic leukemia, acute lymphocytic leukemia and blast phase of chronic myelocytic leukemia. Cytarabine is indicated in combination with [daunorubicin] for the treatment of newly-diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in adults and pediatric patients 1 year and older. FDA Label Intrathecal treatment of lymphomatous meningitis. In the majority of patients such treatment will be part of symptomatic palliation of the disease. Mechanism of Action Cytarabine acts through direct DNA damage and incorporation into DNA. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. It exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and under certain conditions blocking the progression of cells from the G1 phase to the S-phase. Although the mechanism of action is not completely understood, it appears that cytarabine acts through the inhibition of DNA polymerase. A limited, but significant, incorporation of cytarabine into both DNA and RNA has also been reported. Cytarabine is converted intracellularly to the nucleotide, cytarabine triphosphate (ara-CTP, cytosine arabinoside triphosphate). Although the exact mechanism(s) of action of cytarabine has not been fully elucidated, cytarabine triphosphate appears to inhibit DNA polymerase by competing with the physiologic substrate, deoxycytidine triphosphate, resulting in the inhibition of DNA synthesis. Although limited, incorporation of cytarabine triphosphate into DNA and RNA may also contribute to the cytotoxic effects of the drug. Cytarabine is a potent immunosuppressant which can suppress humoral and/or cellular immune responses; however, the drug does not decrease preexisting antibody titers and has no effect on established delayed hypersensitivity reactions. Cytarabine liposome injection is a sustained-release formulation of the active ingredient cytarabine designed for direct administration into the cerebrospinal fluid (CSF). Cytarabine is a cell cycle phase-specific antineoplastic agent, affecting cells only during the S-phase of cell division. Intracellularly, cytarabine is converted into cytarabine-5'-triphosphate (ara-CTP), which is the active metabolite. The mechanism of action is not completely understood, but it appears that ara-CTP acts primarily through inhibition of DNA polymerase. Incorporation into DNA and RNA may also contribute to cytarabine cytotoxicity. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. /Cytarabine liposome injection/ |
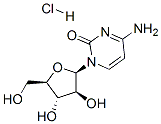
| 分子式 |
C9H14CLN3O5
|
|---|---|
| 分子量 |
279.6776
|
| 精确质量 |
279.062
|
| 元素分析 |
C, 38.65; H, 5.05; Cl, 12.68; N, 15.02; O, 28.60
|
| CAS号 |
69-74-9
|
| 相关CAS号 |
147-94-4 (free); 69-74-9 (HCl)
|
| PubChem CID |
6253
|
| 外观&性状 |
White solid powder
|
| 沸点 |
545.7ºC at 760 mmHg
|
| 熔点 |
197-198 °C(lit.)
|
| 闪点 |
283.8ºC
|
| tPSA |
130.83
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
383
|
| 定义原子立体中心数目 |
4
|
| SMILES |
Cl[H].O1[C@]([H])(C([H])([H])O[H])[C@]([H])([C@@]([H])([C@@]1([H])N1C(N=C(C([H])=C1[H])N([H])[H])=O)O[H])O[H]
|
| InChi Key |
KCURWTAZOZXKSJ-JBMRGDGGSA-N
|
| InChi Code |
InChI=1S/C9H13N3O5.ClH/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8;/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16);1H/t4-,6-,7+,8-;/m1./s1
|
| 化学名 |
4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one;hydrochloride
|
| 别名 |
MK 8242; MK8242; MK-8242; SCH-900242; SCH 900242; SCH900242; aracytidine; cytarabine hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50~55 mg/mL (178.8~196.7 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.94 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5755 mL | 17.8776 mL | 35.7551 mL | |
| 5 mM | 0.7151 mL | 3.5755 mL | 7.1510 mL | |
| 10 mM | 0.3576 mL | 1.7878 mL | 3.5755 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01661881 | Active Recruiting |
Drug: Rituximab Drug: Cytarabine |
Mantle Cell Lymphoma | Dana-Farber Cancer Institute | August 16, 2012 | Phase 2 |
| NCT04330820 | Active Recruiting |
Drug: Venetoclax Oral Tablet | Relapsed Adult AML Refractory AML |
Technische Universität Dresden | April 6, 2020 | Phase 1 Phase 2 |
| NCT03069352 | Active Recruiting |
Drug: Cytarabine Drug: Venetoclax |
Acute Myeloid Leukemia (AML) |
AbbVie | May 23, 2017 | Phase 3 |
| NCT02658487 | Active Recruiting |
Drug: Cytarabine Drug: Vosaroxin |
Myeloid Sarcoma Acute Myeloid Leukemia |
Vanderbilt-Ingram Cancer Center |
March 2016 | Phase 2 |
| NCT04115631 | Active Recruiting |
Drug: Cytarabine Drug: Acalabrutinib |
Mantle Cell Lymphoma Liver Lymphoma |
ECOG-ACRIN Cancer Research Group |
December 13, 2019 | Phase 2 |