| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 7% of ingested mannitol is absorbed during gastrointestinal perfusion in uremic patients. Inhalation of 635 mg of mannitol powder yields a plasma Cmax of 13.71 μg/mL in 1.5 hours (Tmax) and a mean systemic AUC of 73.15 μg\*h/mL. Mannitol is primarily excreted unchanged in the urine. Following oral inhalation of 635 mg of mannitol in healthy volunteers, 55% of the total dose was recovered unchanged in the urine; following oral or intravenous administration of 500 mg, the corresponding values were 54 and 87%, respectively. Mannitol administered intravenously has a volume of distribution of 34.3 L. Intravenous administration of mannitol yields a total clearance of 5.1 L/hr and renal clearance of 4.4 L/hr. MANNITOL IS GENERALLY REGARDED AS BEING UNABSORBED FROM GI TRACT. HOWEVER, RECENT WORK CONTRADICTS THIS BELIEF, FOR 18% OF ORAL DOSE OF D-(14)C MANNITOL WAS RECOVERED UNCHANGED IN 48-HR URINE OF HUMAN SUBJECTS & UP TO 19% AS CO2 IN EXPIRED AIR IN 12 HR. 32% PRESENT IN FECES IN 48 HR...UNABSORBED MATERIAL. SUBSTANCES /MANNITOL/ HAVING APPARENT VOL OF DISTRIBUTION CORRESPONDING TO TOTAL EXTRACELLUR WATER, WHICH IS ABOUT 20% BODY WT...PENETRATE CAPILLARY MEMBRANES BUT DO NOT PENETRATE CELLULAR MEMBRANES. MANNITOL UNDERGOES VERY LITTLE REABSORPTION, & FOR MANY PRACTICAL PURPOSES TUBULE MAY BE CONSIDERED TO BE IMPERMEABLE TO IT. ...OSMOTIC DIURETICS, WHICH, BY DEFINITION, ARE POORLY REABSORBED BY RENAL TUBULES, ARE ALSO NOT ABSORBED FROM GI TRACT. ...THESE AGENTS MUST BE ADMIN PARENTERALLY...TO ACHIEVE EFFECTIVE PLASMA CONCN. Metabolism / Metabolites Mannitol is metabolized only slightly, if at all, to glycogen in the liver. ...POLYHYDRIC SUGAR ALC...MANNITOL (C6H14O6)...LARGELY EXCRETED UNCHANGED IN URINE. MANNITOL OCCURS IN LARGE AMT IN SPORES OF ASPERGILLUS ORYZAE, WHERE IT IS RAPIDLY METABOLIZED IN EARLY STAGES OF GERMINATION. IT IS CONVERTED TO FRUCTOSE BY D-MANNITOL DEHYDROGENASE... ...FATE OF MANNITOL IN ANIMAL BODY (MONKEYS, RABBITS, RATS, DOGS, ETC) AFTER ABSORPTION FROM DIGESTIVE TRACT INCL LIMITED CONVERSION TO GLYCOGEN IN LIVER & ELIMINATION OF BALANCE UNCHANGED IN URINE. Biological Half-Life Mannitol has an elimination half-life of 4.7 hours following oral administration; the mean terminal elimination half-life is similar regardless of administration route (oral, inhalation, and intravenous. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Hydroxyurea (HU) is a potent mammalian teratogen. Within 2-4 hours after maternal injection, HU causes 1) a rapid episode of embryonic cell death and 2) profound inhibition of embryonic DNA synthesis. A variety of antioxidants delays the onset of embryonic cell death and reduces the incidence of birth defects. Antioxidants do not block the inhibition of DNA synthesis, indicating that early embryonic cell death is not caused by inhibited DNA synthesis. We have suggested that some HU molecules may react within the embryo to produce H2O2 and subsequent free radicals, including the very reactive hydroxyl free radical. The free radicals could cause the early cell death; antioxidants are believed to terminate the aberrant free radical reactions resulting in lessened developmental toxicity. To investigate whether hydroxyl free radicals cause the early episode of cell death, pregnant New Zealand white rabbits were injected subcutaneously on gestational day 12 with a teratogenic dose of HU (650 mg/kg) in the presence or absence of 550 mg/kg of D-mannitol (Man), a specific scavenger of hydroxyl free radicals. Osmotic control rabbits received HU plus 550 mg/kg of xylose (Xyl, a nonactive aldose). At term, the teratologic effects of HU were ameliorated by Man as evidenced by decreased incidences of the expected limb malformations. Xyl exerted no demonstrable effect on HU teratogenesis. Histological examination of limb buds at 3-8 hours after maternal injection, showed that Man delayed the onset of HU-induced cell death by as much as 4 hours. Xyl had no effect. That Man acts within the embryo was shown by performing intracoelomic injections on alternate implantation sites with Man, Xyl, or saline followed by subcutaneous injection of the pregnant doe with HU. Embryos were harvested 3-8 hours later. Limb buds from saline- and Xyl-injected embryos exhibited the typical pattern of widespread HU-induced cell death at 3-4 hours, whereas Man-injected embryos did not exhibit cell death until 5-8 hours. These results are consistent with those reported for antioxidant-mediated amelioration of HU-induced developmental toxicity and with the hypothesis that hydroxyl free radicals are the proximate reactive species in HU-induced early embryonic cell death. ... Pentobarbital attenuated the blood-brain barrier disruption induced by hyperosmolar mannitol. This may be attributed, at least in part, to the blood pressure effect of pentobarbital. Implications: When the blood-brain barrier (BBB) was disrupted by a hyperosmolar solution, pentobarbital attenuated the degree of leakage of the blood-brain barrier. Systemic hypotension caused by pentobarbital played a significant role in decreasing the leakage. Our study suggests that when the blood-brain barrier is disrupted, pentobarbital may be effective in protecting the blood-brain barrier. Furthermore, systemic blood pressure plays an important role in determining the degree of disruption. Non-Human Toxicity Values LD50 Rat oral 13,500 mg/kg LD50 Rat iv 9690 mg/kg LD50 Mouse oral 22 g/kg LD50 Mouse ip 14 g/kg LD50 Mouse iv 7470 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Diuretics, Osmotic MEDICATION (VET): INTRAAORTIC PRETREATMENT WITH 10% MANNITOL SOLN PRIOR TO INTRAAORTIC CONTRAST ANGIOGRAPHY PROCEDURES GIVES RENAL PROTECTION & REDUCES INCIDENCE OF PARAPLEGIA & AZOTEMIA IN DOGS. MEDICATION (VET): IN DOGS AS OSMOTIC DIURETIC CAUSING CELLULAR DEHYDRATION, TO REDUCE INTRAOCULAR PRESSURE IN GLAUCOMA, & TO REDUCE CEREBRAL EDEMA FOLLOWING SURGERY OR INJURY. DOSE FOR REDUCTION OF INTRACRANIAL PRESSURE & BRAIN MASS PRIOR TO NEUROSURGERY, OR FOR REDUCTION OF INTRAOCULAR TENSION...OF CONGESTIVE GLAUCOMA OR FOR OPHTHALMIC SURGERY, IS 1.5 TO 2 G/KG, GIVEN AS 15 OR 20% SOLN OVER PERIOD OF 30 TO 60 MIN. For more Therapeutic Uses (Complete) data for D-MANNITOL (15 total), please visit the HSDB record page. Drug Warnings IN EDEMATOUS STATES ASSOC WITH DIMINISHED CARDIAC RESERVE, ADMIN OF MANNITOL INTRODUCES A RISK THAT MAY FAR OUTWEIGH ANY THERAPEUTIC BENEFIT. CONTRAINDICATIONS TO ADMIN OF MANNITOL INCL RENAL DISEASE...ANURIA, MARKED PULMONARY CONGESTION OR EDEMA, MARKED DEHYDRATION, & INTRACRANIAL HEMORRHAGE... MANNITOL SHOULD BE TERMINATED IF PATIENTS DEVELOPS...PROGRESSIVE RENAL DYSFUNCTION, HEART FAILURE, OR PULMONARY CONGESTION. ITS SAFE USE DURING PREGNANCY & IN CHILDREN UNDER 12 YR OF AGE HAS NOT BEEN ESTABLISHED. FACTITIOUS HYPOPHOSPHATEMIA WAS OBSERVED IN A PATIENT RECEIVING LARGE AMT OF IV MANNITOL. CONCN AS LOW AS 25 MMOL/L INHIBITED PHOSPHORUS MEASUREMENT BY DUPONT ACA ENDPOINT METHOD; A KINETIC METHOD WAS UNAFFECTED. MECHANISM OF MANNITOL INTERFERENCE WAS BINDING TO MOLYBDATE IN REACTION, DECR RATE OF COLOR DEVELOPMENT & ENDPOINT MEASUREMENT. For more Drug Warnings (Complete) data for D-MANNITOL (9 total), please visit the HSDB record page. Pharmacodynamics Chemically, mannitol is an alcohol and a sugar, or a polyol; it is similar to xylitol or sorbitol. However, mannitol has a tendency to lose a hydrogen ion in aqueous solutions, which causes the solution to become acidic. For this reason, it is not uncommon to add a substance to adjust its pH, such as sodium bicarbonate. Mannitol is commonly used to increase urine production (diuretic). It is also used to treat or prevent medical conditions that are caused by an increase in body fluids/water (e.g., cerebral edema, glaucoma, kidney failure). Mannitol is frequently given along with other diuretics (e.g., furosemide, chlorothiazide) and/or IV fluid replacement. Inhaled mannitol has the possibility to cause bronchospasm and hemoptysis; the occurrence of either should lead to discontinuation of inhaled mannitol. |
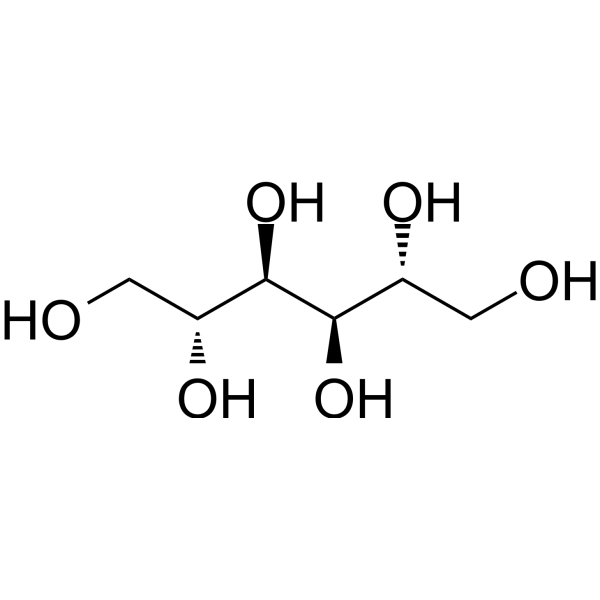
| 分子式 |
C6H14O6
|
|---|---|
| 分子量 |
182.1718
|
| 精确质量 |
182.079
|
| CAS号 |
69-65-8
|
| 相关CAS号 |
D-Mannitol-d8;D-Mannitol-13C;132202-29-0;D-Mannitol-13C6;287112-34-9;D-Mannitol-2-13C;287100-69-0;D-Mannitol-d2;2649096-16-0;D-Mannitol-d;75607-68-0;D-Mannitol-13C,d2;1217463-58-5
|
| PubChem CID |
6251
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
494.9±0.0 °C at 760 mmHg
|
| 熔点 |
167-170ºC
|
| 闪点 |
292.5±23.3 °C
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
| 折射率 |
1.597
|
| LogP |
-4.67
|
| tPSA |
121.38
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
105
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C([C@H]([C@H]([C@@H]([C@@H](CO)O)O)O)O)O
|
| InChi Key |
FBPFZTCFMRRESA-KVTDHHQDSA-N
|
| InChi Code |
InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4-,5-,6-/m1/s1
|
| 化学名 |
(2R,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 36 mg/mL (~197.62 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (548.94 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.4894 mL | 27.4469 mL | 54.8938 mL | |
| 5 mM | 1.0979 mL | 5.4894 mL | 10.9788 mL | |
| 10 mM | 0.5489 mL | 2.7447 mL | 5.4894 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Cerebrum and Cardiac Protection With Allopurinol in Neonates With Critical Congenital Heart Disease Requiring Cardiac Surgery With Cardiopulmonary Bypass
CTID: NCT04217421
Phase: Phase 3 Status: Recruiting
Date: 2024-05-16