| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
Cholesteryl ester transfer protein (CETP); recombinant human (rh) CETP (IC50 = 204.6 nM)[1]; human plasma CETP (IC50 = 6 μM)[2]
Cholesteryl Ester Transfer Protein (CETP) (IC50 = 0.2 μM) [2] Cholesteryl Ester Transfer Protein (CETP) (1 μM concentration inhibits human plasma CETP activity by 85%) [1] |
|---|---|
| 体外研究 (In Vitro) |
Dalcetrapib (JTT-705)(0.1-10 μM;21 小时)以剂量依赖性方式增强前 β-HDL 的产生[1]。在 HepG2 中,Dalcetrapib(0-30 μM;24 小时)剂量依赖性地抑制培养基的 CETP 活性[3]。
使用选择性靶向CETP的药物(dalcetrapib, torcetrapib, anacetrapib)研究了胆固醇酯转移蛋白(CETP)活性影响HDL代谢的机制。与torcetrapib和anacetrapib相比,dalcetrapib需要半胱氨酸13来降低CETP活性,以胆固醇酯(CE)从HDL到LDL的转移来测量,并且不影响CE从HDL3到HDL2的转移。只有dalcetrapib在体外人血浆中引起CETP的构象变化,在体内也观察到并与CETP活性相关。dalcetrapib≤3µM时,cetp诱导的体外人血浆中pre-β-HDL的形成没有变化,在10µM时增加。torcetrapib和anacetrapib(0.1 ~ 10µM)对前β- hdl形成的剂量依赖性抑制表明dalcetrapib调节CETP活性。[1] 1 μM浓度下抑制人血浆CETP活性达85%,并使pre-β-HDL形成增加2.3倍 [1] 1 μM浓度处理HepG2细胞24小时后,apoA-I介导的胆固醇流出增加50% [3] 1 μM浓度处理HepG2细胞24小时,使HDL合成相关基因ABC1的表达上调1.8倍 [3] 体外可强效抑制CETP介导的HDL与LDL之间的胆固醇酯转移 [2] |
| 体内研究 (In Vivo) |
在兔子中,dacletrapib (JTT-705)(30 或 100 mg/kg;口服;每天一次,连续三天)显着升高血浆 HDL 胆固醇[2]。给予 dacletrapib(100 mg/kg;ir;每天两次,持续 7 天)后,粪便中的中性甾醇、胆汁酸和血浆 HDL-胆固醇显着增加[1]。
在给仓鼠注射[3H]胆固醇标记的自体巨噬细胞,并给予dalcetrapib (100 mg,每日2次)、torcetrapib [30 mg,每日1次(QD)]或anacetrapib (30 mg, QD)的实验中,只有dalcetrapib显著增加了[3H]中性固醇和[3H]胆汁酸的粪便消除,而所有化合物都增加了血浆HDL-[3H]胆固醇。这些数据表明dalcetrapib对CETP活性的调节不会抑制CETP诱导的pre-β-HDL的形成,这可能是增加逆向胆固醇运输所必需的。[1] 对C57BL/6小鼠口服给药30 mg/kg/天,连续7天,血浆CETP活性降低60%,HDL-C水平升高40% [1] |
| 酶活实验 |
Dalcetrapib与Cys13的选择性结合[1]
采用定点诱变的方法构建了含有丝氨酸残基代替Cys13的CETP (C13S CETP)。该蛋白通过大规模瞬时转染在HEK293EBNA细胞中表达,并按照下面描述的方法纯化重组人(rh)CETP。 cetp和C13S介导的CE从HDL向LDL转移的抑制作用[1] 采用闪烁接近测定试剂盒测定dalcetrapib、torcetrapib和anacetrapib通过rhCETP和C13S CETP降低CE从HDL向LDL转移的抑制效力(IC50)。简单地说,将[3H] ce标记的HDL供体颗粒与纯化的CETP蛋白(终浓度0.5µg/ml)和生物素化的LDL受体颗粒在37℃下孵育3小时。随后,加入含有选择性结合生物素化LDL的液体闪烁鸡尾酒的链霉亲和素偶联聚乙烯烯珠,通过β计数测量[3H]CE分子转移到LDL的量。 CE从HDL3向HDL2转移的抑制作用[1] 如前所述,使用放射性标记脂质转移法评估HDL亚组分之间的脂质运动。脂蛋白亚组分(d > 1.063 g/ml)用[3H]CE标记。[3H]连续超离心制备ce标记HDL3 (1.125 < d < 1.210 g/ml)。[3H] ce标记的HDL3和非放射性标记的HDL2 (1.063 < d < 1.125 g/ml)在相同的磷脂基础上添加(2.3 μg/管)。在1% BSA、21 mM tris-HCl (pH 7.4)、0.5% NaCl和0.006% EDTA的条件下(含或不含rhCETP (0.5 μg/管))培养脂蛋白混合物。Dalcetrapib、torcetrapib和anactrapib在0.001、0.01、0.1、1和10µM的浓度下测试,总容积为0.715 ml,在37℃下孵育4 h。孵育后,在4℃超离心(d = 1.125 g/ml) 19 h分离HDL2和HDL3组分。通过闪烁计数测量HDL2(上层)和HDL3(下层)亚组分的总放射性。CETP活性表示为HDL2部分中总放射性恢复的百分比。 化合物在CETP上的结合位点:葡萄糖固定化rhCETP上的结合位点竞争[1] 根据Connolly等人的研究,结合研究使用Weinberg等人描述的细胞系表达的rhCETP,并通过疏水相互作用层析和大小排除层析(SEC)纯化。将BSA和rhCETP固定在cnbr活化的sepharoseTM 4 Fast Flow上。 测定了300 pmol固定化rhCETP (3 μM)或相同质量的BSA分别与0.25 μM [14C]torcetrapib或2.5 μM [14C]dalcetrapib混合,以及总体积为100 μl的未标记CETP抑制剂,与放射性化合物的竞争(共孵育实验)和预孵育后的位移(后者加或不加还原剂tris(2-羧基乙基)膦(TCEP))。[14C]dalcetrapib在用CETP孵育前用胰脂肪酶处理生成[14C]dalcetrapib-硫醇。用闪烁计数法测定了与蔗糖结合的放射性。 体外CETP活性。[2] 体外CETP活性由[3H]胆固醇酯从HDL向载脂蛋白b转移的速率测定。13,14正常血脂志愿者的血液被收集到装有肝素的管子中。4°C, 1500g离心15 ~ 30min分离血浆。将每种测试化合物适量溶于n -甲基-2-吡咯烷酮和聚乙二醇(平均分子量为400)的1:1混合物中,加入血浆中。有机溶剂的最终浓度为2%。与含[3H]CE的HDL反应混合物在37℃下孵育4 h后,用硫酸葡聚糖和氯化镁(终浓度分别为0.075%和37.5 mM)沉淀含载脂蛋白b的脂蛋白,取100 μL含HDL的上清进行放射性计数。CETP介导的转移是通过上清的放射性降低来确定的,从5个剂量获得的剂量-反应曲线估计达到50% CETP活性抑制的浓度。 将重组CETP与底物(HDL中的胆固醇酯和LDL中的甘油三酯)在缓冲液中孵育,加入系列稀释的Dalcetrapib(JTT-705, RO4607381) 孵育后检测胆固醇酯向LDL的转移情况,计算抑制率和IC50值 [2] 将人血浆CETP与荧光标记的胆固醇酯底物共同孵育,向反应体系中加入Dalcetrapib(JTT-705, RO4607381) 通过检测荧光强度变化,评估对CETP活性的抑制作用 [1] |
| 细胞实验 |
CETP ODNs和CETP化学抑制剂Dalcetrapib (JTT-705)递送[3]
将HepG2细胞置于6孔板中转染。通过阳离子脂质体Tfx-20将odn传递到培养的HepG2细胞中。将正义或反义odn和脂质体(12 μM)混合在Opti-MEM中。将70-80%汇合的培养细胞用PBS冲洗2次,然后加入odn -脂质体混合物。细胞在37℃、5% CO2、100%湿度条件下暴露12 h后返回生长培养基。孵育8 h后,用PBS冲洗细胞2次,制备总RNA和细胞蛋白。孵育24 h后,收集生长培养基和细胞进行CETP质量测定和蛋白定量。测定不同浓度的odn (0 ~ 8 μM)。 HepG2细胞于6孔板中,培养至70-80%的合度。PBS洗涤后,细胞与不同浓度(0-30 μM)的化学抑制剂Dalcetrapib (JTT-705) 生长培养基孵育,在2% DMSO中溶解24 h。RT-PCR采用总RNA法。 这些elisa应用于22名健康受试者的血浆样本,这些受试者接受单次口服剂量600 mg dalcetrapib。分别在服用达西trapib前、摄入后2、4、6、8、12和24 h采集血浆样本。Dalcetrapib (JTT-705)水平按照其他文献的描述进行测定,CETP免疫反应性采用上述ELISA测定,JHC-1或6/2为捕获抗体,6/6或6/17为检测抗体。体外CETP活性测定试剂盒测定CETP活性[1]。 ELISA定量前β - hdl。添加或未添加rhCETP的样品在torcetrapib、anacetrapib和Dalcetrapib (JTT-705)(0.10µM至10µM)存在下孵育21 h。如前所述,采用ELISA法测定β- hdl前浓度[1]。 将HepG2细胞接种到6孔板中,培养至80%融合度 向细胞中加入系列稀释的Dalcetrapib(JTT-705, RO4607381),孵育24小时 检测胆固醇流出率,并通过RT-PCR测定ABC1的mRNA表达水平 [3] Animal Protocol: 实验选用雄性C57BL/6小鼠(8周龄) 将Dalcetrapib(JTT-705, RO4607381)溶于0.5%羧甲基纤维素钠中,以30 mg/kg/天的剂量通过口服灌胃给药,连续7天 末次给药后24小时收集血浆样本,检测CETP活性和脂质谱 [1] |
| 动物实验 |
Animal/Disease Models: Male JW rabbits[2]
Doses: 30 or 100 mg/kg Route of Administration: Oral administration, one time/day for 3 days Experimental Results: Increased plasma HDL cholesterol by 27% and 54% at 30 mg/kg and 100 mg/kg, respectively. In vivo RCT study.[1] To investigate the effect of dalcetrapib, torcetrapib, and anacetrapib on macrophage-to-feces RCT, radiolabeled macrophages from the peritoneal cavity of donor Golden Syrian hamsters preinjected with [3H]cholesterol were prepared as previously described. Male recipient Golden Syrian hamsters, 8 weeks old, on a standard chow diet were preadministered dalcetrapib [100 mg/kg twice daily (BID)], torcetrapib [30 mg/kg once daily (QD)], anacetrapib (30 mg/kg QD), or vehicle (0.5% methylcellulose BID) for 7 days by oral gavage before intraperitoneal injection of [3H]cholesterol-labeled macrophages (3.8 × 106 cells/90.6 kBq/0.5 ml per animal) at day 0. The percentage of esterified cholesterol in injected macrophages was 21% (mass) and 16% (labeled). Animals continued to receive vehicle or test compounds daily for 10 days. Samples for plasma lipid analysis were obtained on days −7, 0, 3, 7, and 10 and for radioactivity levels on days 3, 7, and 10. Total cholesterol and HDL-C were measured by enzymatic methods. HDL-C was measured as the cholesterol concentration in the HDL fraction separated by polyethylene glycol 6000 solution. The area under the plasma HDL-C concentration-time curve (HDL-C·AUC) during the RCT study period (day 0 to day 10) was calculated from plasma HDL-C levels (at day 0, 3, 7, and 10) by the trapezoidal method. Ex Vivo CETP Activity. [2] Ex vivo CETP activity was measured in whole plasma obtained from male JW rabbits (11−15 weeks old), which were orally administered the test compounds (10 or 30 mg/kg). 15 Plasma was isolated by centrifugation at 11000g for 5 min at 4 °C. Whole plasma (1.8 μL) from control or treated rabbits was diluted to 600 μL with Tris-buffered saline (pH 7.4) containing 0.1 mg/mL of bovine serum albumin and was incubated at 37 °C for 15 h in the presence of HDL containing [3H]CE and VLDL/LDL. After precipitation of VLDL/LDL using dextran sulfate and magnesium chloride, the radioactivity of the supernatant containing HDL was counted. CETP activity was determined by the decrease in radioactivity versus that of the blank sample without plasma. In Vivo Plasma HDL Cholesterol Level. HDL cholesterol levels were measured in the plasma after precipitation of apoprotein B-containing lipoproteins with 13% poly(ethylene glycol) (at an average molecular weight of 6000) by an enzyme assay. Plasma was obtained from male JW rabbits (11−15 weeks old, N = 5), which were orally administered compound 27/Dalcetrapib (JTT-705) (30 or 100 mg/kg) once a day for 3 days.[2] |
| 参考文献 |
|
| 其他信息 |
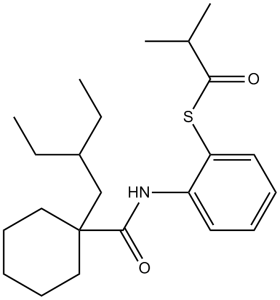
2-methylpropanethioic acid S-[2-[[[1-(2-ethylbutyl)cyclohexyl]-oxomethyl]amino]phenyl] ester is an anilide.
Dalcetrapib is under investigation for the treatment of Acute Coronary Syndrome. Dalcetrapib is a cholesteryl ester transfer protein (CETP) inhibitor that can produce an increase in serum HDL-cholesterol levels and a decrease in serum LDL-cholesterol levels. Dalcetrapib is structurally dissimilar from other CETP inhibitors, including torcetrapib, and raises functional HDL by a different mechanism. Drug Indication Lipoprotein deficiency Dalcetrapib (JTT-705, RO4607381) is a selective CETP inhibitor that modulates CETP activity to maintain efficient pre-β-HDL formation [1] Its mechanism of action involves inhibiting the transfer of cholesteryl esters between HDL and other lipoproteins, thereby increasing HDL-C levels and promoting reverse cholesterol transport [1] It exerts dual regulatory effects on HDL metabolism in HepG2 cells, including enhancing cholesterol efflux and upregulating HDL synthesis-related gene expression [3] |
| 分子式 |
C23H35NO2S
|
|
|---|---|---|
| 分子量 |
389.59
|
|
| 精确质量 |
389.239
|
|
| 元素分析 |
C, 70.91; H, 9.06; N, 3.60; O, 8.21; S, 8.23
|
|
| CAS号 |
211513-37-0
|
|
| 相关CAS号 |
|
|
| PubChem CID |
6918540
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.066 g/cm3
|
|
| 沸点 |
528.912ºC at 760 mmHg
|
|
| 闪点 |
273.676ºC
|
|
| LogP |
6.749
|
|
| tPSA |
71.47
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
481
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
YZQLWPMZQVHJED-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C23H35NO2S/c1-5-18(6-2)16-23(14-10-7-11-15-23)22(26)24-19-12-8-9-13-20(19)27-21(25)17(3)4/h8-9,12-13,17-18H,5-7,10-11,14-16H2,1-4H3,(H,24,26)
|
|
| 化学名 |
S-[2-[[1-(2-ethylbutyl)cyclohexanecarbonyl]amino]phenyl] 2-methylpropanethioate
|
|
| 别名 |
Dalcetrapib; JTT-705; RO-4607381; RO4607381; 211513-37-0; Dalcetrapib (JTT-705, RO4607381); S-[2-[[1-(2-ethylbutyl)cyclohexanecarbonyl]amino]phenyl] 2-methylpropanethioate; JTT705;RO 4607381; JTT705; JTT 705
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.42 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.42 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.42 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% methylcellulose: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5668 mL | 12.8340 mL | 25.6680 mL | |
| 5 mM | 0.5134 mL | 2.5668 mL | 5.1336 mL | |
| 10 mM | 0.2567 mL | 1.2834 mL | 2.5668 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04676867 | Completed Has Results | Drug: Dalcetrapib Other: Placebo |
Covid19 | DalCor Pharmaceuticals | January 11, 2021 | Phase 2 |
| NCT05918861 | Recruiting | Drug: Dalcetrapib Drug: Placebo |
Acute Coronary Syndrome | DalCor Pharmaceuticals | October 3, 2023 | Phase 3 |
| NCT01363999 | Completed Has Results | Drug: atorvastatin Drug: dalcetrapib |
Healthy Volunteer | Hoffmann-La Roche | June 2011 | Phase 1 |
| NCT01516541 | Completed | Drug: Placebo Drug: dalcetrapib |
Cardiovascular Disease, Coronary Heart Disease, Dyslipidemia, Peripheral Arterial Disease (PAD) |
Hoffmann-La Roche | January 2012 | Phase 3 |