| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
Antibacterial
|
|---|---|
| 体外研究 (In Vitro) |
链阳菌素 B(奎奴普汀)和链阳菌素 A(达福普汀)以 30:70 的比例组合而成奎奴普汀/达福普汀 (Q/D)[1]。奎奴普汀/达福普汀是一种半合成注射用链阳菌素,以 30:70 的重量比结合了两种协同抗生素成分奎奴普汀(B 型链阳菌素)和达福普汀(A 型链阳菌素)。两种成分均源自普那霉素[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Quinupristin and dalfopristin is distributed into milk in rats ... . The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. In rats and monkeys quinupristin and dalfopristin undergo rapid elimination from the blood and wide tissue distribution. Nevertheless, they do not penetrate the central nervous system or cross the placenta to any significant degree and they do not appear to be subject to significant body retention following cessation of administration. The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. Both compounds are primarily eliminated through the bile into the faeces; quinupristin is mainly excreted unchanged whereas dalfopristin is extensively metabolized beforehand. The metabolites include the microbiologically active pristinamycin PIIA for dalfopristin and the microbiologically active glutathione- and cysteine-conjugated derivatives for quinupristin. Quinupristin and dalfopristin appear to be handled in a similar manner by humans. Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. The pharmacokinetic profile of quinupristin is dose-independent and so is that of dalfopristin and RP 12536 when considered together. Extravascular diffusion of quinupristin/dalfopristin has been assessed in human non-inflammatory interstitial fluid. Fecal excretion constitutes the main elimination route for both parent drugs and their metabolites (75 to 77% of dose). Urinary excretion accounts for approximately 15% of the quinupristin and 19% of the dalfopristin dose. Preclinical data in rats have demonstrated that approximately 80% of the dose is excreted in the bile and suggest that in man, biliary excretion is probably the principal route for fecal elimination. Metabolism / Metabolites Converted to an active non-conjugated metabolite by hydrolysis. Quinupristin and dalfopristin are converted to several major active metabolites: 2 conjugated (with glutathione and cysteine) metabolites for quinupristin and one nonconjugated (formed by hydrolysis) metabolite for dalfopristin, which also act synergistically with the complementary parent drug. This conversion occurs in vitro by nonenzymatic reactions independent of cytochrome P-450 (CYP) and glutathione transferase enzymes. Biological Half-Life The elimination half-life is approximately 0.70 hours. The elimination half-life of quinupristin and dalfopristin is approximately 0.85 and 0.70 hours, respectively. The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. ... The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. ... Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Moderate Interactions Concomitant administration of Synercid and nifedipine (repeated oral doses) and midazolam (intravenous bolus dose) in healthy volunteers led to elevated plasma concentrations of these drugs. The Cmax increased by 18% and 14% (median values) and the AUC increased by 44% and 33% for nifedipine and midazolam, respectively. In vitro drug interaction studies have demonstrated that Synercid significantly inhibits cytochrome P450 3A4 metabolism of cyclosporin A, midazolam, nifedipine and terfenadine. In addition, 24 subjects given Synercid 7.5 mg/kg q8h for 2 days and 300 mg of cyclosporine on day 3 showed an increase of 63% in the AUC of cyclosporine, an increase of 30% in the Cmax of cyclosporine, a 77% increase in the half life of cyclosporine, and, a decrease of 34% in the clearance of cyclosporine. Therapeutic level monitoring of cyclosporine should be performed when cyclosporine must be used concomitantly with Synercid. A drug interaction between Synercid and digoxin cannot be excluded but is unlikely to occur via CYP3A4 enzyme inhibition. Synercid has shown in vitro activity (MICs of 0.25 ug/mL when tested on two strains) against Eubacterium lentum. Digoxin is metabolized in part by bacteria in the gut and as such, a drug interaction based on Synercid's inhibition of digoxin's gut metabolism (by Eubacterium lentum) may be possible. A case is presented in which a 21-yr-old woman who was receiving 150 mg/day oral cyclosporine after kidney transplantation developed elevated cyclosporine blood levels 2 days after starting treatment with intravenous injections of 20 mg/kg/day quinupristin/dalfopristin. Baseline trough cyclosporine levels ranged from 80 to 105 ng/ml. Two and 3 days after initiation of quinupristin/dalfopristin therapy, trough cyclosporine levels increased to 261 and 291 ng/ml, respectively. The cyclosporine dosage was decreased to 100 mg/day and the blood levels returned to baseline. After discontinuation of quinupristin/dalfopristin, the cyclosporine blood concentration decreased and the dosage was increased to the previous regimen. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anti-Bacterial Agents Quinupristin and dalfopristin is used IV in adults for the treatment of serious or life-threatening infections caused by susceptible strains of vancomycin-resistant Enterococcus faecium (VREF), including infections associated with VREF bacteremia. Quinupristin and dalfopristin became commercially available in the US for this indication under the principles and procedures of FDA's accelerated review process that allows approval based on analysis of surrogate markers of response (i.e., clearance of bacteremia), rather than clinical end points such as cure of infection or survival. Controlled clinical studies are underway to confirm the validity of this surrogate marker. /Included in US product labeling/ Quinupristin and dalfopristin is used IV for the treatment of complicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible strains) or Streptococcus pyogenes (group A beta-hemolytic streptococci). /Included in US product labeling/ The semi-synthetic streptogramin quinupristin/dalfopristin antibiotic exerts potent bactericidal activity against Staphylococcus aureus. /The researchers/ investigated whether, like other bactericidal antibiotics used at subinhibitory concentrations, quinupristin/dalfopristin enhances release of toxins by Gram-positive cocci. The activity of quinupristin/dalfopristin on exotoxin release by S. aureus was investigated by 2D SDS-PAGE combined with MALDI-TOF/MS analysis and by western blotting. /The researchers/ show that quinupristin/dalfopristin at subinhibitory concentrations reduces the release of S. aureus factors that induce tumour necrosis factor secretion in macrophages. Furthermore, quinupristin/dalfopristin but not linezolid attenuated S. aureus-mediated killing of infected host cells. When added to S. aureus cultures at different stages of bacterial growth, quinupristin/dalfopristin reduced in a dose-dependent manner the release of specific virulence factors (e.g. autolysin, protein A, alpha- and beta-haemolysins, lipases). In contrast, other presumably non-toxic exoproteins remained unchanged. The results of the present study suggest that subinhibitory quinupristin/dalfopristin inhibits virulence factor release by S. aureus, which might be especially helpful for the treatment of S. aureus infections, where both bactericidal as well as anti-toxin activity may be advantageous. Drug Warnings Adverse venous effects (e.g., thrombophlebitis, pain) may occur; therefore, flush infusion lines with 5% dextrose injection following completion of peripheral infusions with quinupristin and dalfopristin. Do not flush with sodium chloride injection or heparin solutions because of possible incompatibilities. Recommended measures for moderate-to-severe reactions include increasing the infusion volume, changing infusion sites, or establishing central venous access. Concomitant hydrocortisone or diphenhydramine did not alleviate adverse venous effects during clinical studies. Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible. Because Clostridium difficile-associated diarrhea and colitis has been reported with quinupristin and dalfopristin, ranging in severity from mild to life-threatening, it should be considered in the differential diagnosis of patients who develop diarrhea during or following therapy with the drug. To determine whether myalgias/arthralgias occurring in cancer patients who receive quinupristin/dalfopristin are associated with biliary tract dysfunction, 56 patients with vancomycin-resistant enterococcal infections who were treated with quinupristin/dalfopristin 7.5 mg/kg every 8 hr for a mean duration of 12 days (range 2-52 days) /were studied/. Liver function tests, including a test for alkaline phosphatase, were performed before, during and after the end of therapy. All patients were followed for 1 month after completion of therapy. Thirty-eight (68%) of the 56 patients responded. Myalgias/arthralgias were the leading adverse events occurring in 20 (36%) of the patients. Patients with myalgias/arthralgias had significantly higher levels of alkaline phosphatase (mean 318.7 IU/L) during the mid-term therapy cycle compared with patients without any joint or muscular pain (mean 216.3 IU/L, P = 0.05). In addition, 3/18 (16.6%) patients with myalgias/arthralgias had more than five-fold the normal levels of alkaline phosphatase, which did not occur in any of the other patients who did not develop myalgias/arthralgias (P = 0.04). All myalgias/arthralgias resolved after the discontinuation of quinupristin/dalfopristin. By univariate analysis, other factors associated with myalgias/arthralgias were relapse of hematological malignancy (P = 0.01), receiving tacrolimus within 1 month prior to treatment (P = 0.04) and receiving methotrexate during antimicrobial therapy (P = 0.05). Myalgias/arthralgias occur frequently in cancer patients receiving quinupristin/dalfopristin and may be associated with biliary tract dysfunction, as measured by alkaline phosphatase or other factors that could lead to intra-hepatic cholestasis, such as relapse of haematological malignancy or treatment with tacrolimus or methotrexate. For more Drug Warnings (Complete) data for Dalfopristin (13 total), please visit the HSDB record page. Pharmacodynamics Dalfopristin is a streptogramin antibiotic, derived from pristinamycin IIA. |
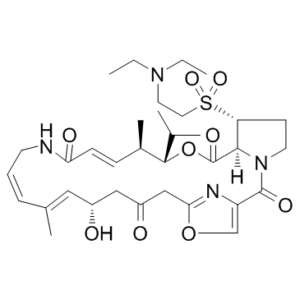
| 分子式 |
C34H50N4O9S
|
|
|---|---|---|
| 分子量 |
690.85
|
|
| 精确质量 |
690.33
|
|
| 元素分析 |
C, 59.11; H, 7.30; N, 8.11; O, 20.84; S, 4.64
|
|
| CAS号 |
112362-50-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
6323289
|
|
| 外观&性状 |
Slightly yellow to yellow powder
White solid |
|
| 密度 |
1.27g/cm3
|
|
| 沸点 |
940.5ºC at 760 mmHg
|
|
| 熔点 |
approximately 150 °C
|
|
| 闪点 |
522.6ºC
|
|
| 蒸汽压 |
0mmHg at 25°C
|
|
| 折射率 |
1.575
|
|
| LogP |
3.616
|
|
| tPSA |
184.8
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
48
|
|
| 分子复杂度/Complexity |
1340
|
|
| 定义原子立体中心数目 |
5
|
|
| SMILES |
S(C([H])([H])C([H])([H])[N+]([H])(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])[H])([C@]1([H])C([H])([H])C([H])([H])N2C(C3=C([H])OC(C([H])([H])C(C([H])([H])[C@@]([H])(C([H])=C(C([H])([H])[H])C([H])=C([H])C([H])([H])N([H])C(C([H])=C([H])[C@@]([H])(C([H])([H])[H])[C@@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])OC(C21[H])=O)=O)O[H])=O)=N3)=O)(=O)=O.S(C([H])([H])[H])(=O)(=O)[O-] |c:48,65,t:55|
|
|
| InChi Key |
SUYRLXYYZQTJHF-VMBLUXKRSA-N
|
|
| InChi Code |
InChI=1S/C34H50N4O9S/c1-7-37(8-2)16-17-48(44,45)28-13-15-38-31(28)34(43)47-32(22(3)4)24(6)11-12-29(41)35-14-9-10-23(5)18-25(39)19-26(40)20-30-36-27(21-46-30)33(38)42/h9-12,18,21-22,24-25,28,31-32,39H,7-8,13-17,19-20H2,1-6H3,(H,35,41)/b10-9+,12-11+,23-18+/t24-,25-,28-,31-,32-/m1/s1
|
|
| 化学名 |
(12Z,32S,33R,6R,7R,8E,13E,15E,17S)-33-((2-(diethylamino)ethyl)sulfonyl)-17-hydroxy-6-isopropyl-7,15-dimethyl-5-oxa-11-aza-1(4,2)-oxazola-3(1,2)-pyrrolidinacycloicosaphane-8,13,15-triene-2,4,10,19-tetraone
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100~125 mg/mL ( 144.74~180.94 mM )
Ethanol : ~100 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (3.01 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4475 mL | 7.2375 mL | 14.4749 mL | |
| 5 mM | 0.2895 mL | 1.4475 mL | 2.8950 mL | |
| 10 mM | 0.1447 mL | 0.7237 mL | 1.4475 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。