| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
hSGLT2 ( EC50 = 1.1 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:达格列净对 hSGLT1 不敏感,IC50 为 1200 倍。达格列净对抗 hSGLT2 的效力比根皮苷强 32 倍,但对抗 hSGLT1 的效力比根皮苷低 4 倍。达格列净对 GLUT 转运蛋白具有高度选择性,在 20 μM 的无蛋白缓冲液中显示出 8-9% 的抑制作用,而在 4% 牛血清白蛋白存在的情况下几乎没有抑制作用。 Dapagliflozin 对 Caco-2 细胞膜具有良好的渗透性,是 P-糖蛋白 (P-gp) 的底物,但不是重要的 P-gp 抑制剂。 10 μM 的达格列净在大鼠、狗、猴和人血清中稳定。达格列净对人 P450 酶没有抑制反应或诱导作用。达格列净的体外代谢途径为葡萄糖醛酸化、羟基化和 O-脱乙基化 激酶测定:达格列净的 hSGLT2 的 EC50 值为 1.1 nM,hSGLT1 的 EC50 值为 1.4 μM,对应于 SGLT2 的 1200 倍选择性,而根皮苷的选择性为 10 倍。达格列净对大鼠 SGLT (rSGLT)2 和 hSGLT2 的抑制效力相当,但与 rSGLT1 相比,达格列净对 rSGLT2 的选择性下降至 200 倍 细胞测定:为了进行细胞存活测定,在与载体或达格列净一起孵育 24 小时后收集细胞预处理30分钟缺血,并用台盼蓝染色对存活细胞进行计数。存活百分比通过将处理的细胞的相对存活数除以未处理的细胞的存活数进行量化来确定。
体外活性。[1] 达格列净对hSGLT2的平均EC50为1.12 nmol/l,而根皮苷的EC50为35.6 nmol/l(表1)。与hSGLT1相比,达格列净和根皮苷的平均EC50值分别为1391和330 nmol/l,表明达格列净对hSGLT2的选择性很高(约1200倍)。达格列净对hSGLT2的效力是根皮苷的32倍,对hSGL41的效力比根皮苷低4倍。Dapagliflozin也是rSGLT2的强效选择性抑制剂,显示平均EC50值为3.0 nmol/l,与rSGLT1相比选择性约为200倍。在人脂肪细胞中检测到,达格列净对GLUT转运蛋白具有高度选择性,在20μmol/l的无蛋白缓冲液中显示出8-9%的抑制作用,在4%牛血清白蛋白存在的情况下几乎没有抑制作用(表2)。将蛋白质添加到该测定中以模拟血浆蛋白结合的体内条件。Phlorizin最低限度地抑制脂肪细胞GLUT活性;然而,根皮苷的苷元根皮素抑制GLUT活性约77%,无论检测中是否存在牛血清白蛋白。 细胞内镁浓度[5] 孵育24小时后,测量并计算细胞内镁浓度。如表3和图3所示,与第一次测量时(0分钟)的对照组相比,用达格列净治疗与细胞内镁浓度增加60%有关。单独使用AG1478和间充质治疗显著降低了细胞内镁浓度。达格列净与AG1478或间充质联合使用可降低镁浓度(均p<0.05)。在120分钟内,除80分钟外,达格列嗪组的镁浓度高于对照组。单独使用AG1478治疗在20、60、100和120分钟时与显著降低的浓度相关。单独使用间充质治疗在整个120分钟期间降低了镁浓度,但80分钟除外。联合治疗(达格列嗪与AG1478和达格列嗪与间充质)在大多数时间点显著降低了镁含量。 |
| 体内研究 (In Vivo) |
在高血糖链脲佐菌素 (STZ) 大鼠中口服 0.1 mg/kg 剂量后,达格列净可将血糖水平降低 55%,部分原因是 C-葡萄糖苷键赋予的代谢稳定性。达格列净表现出良好的吸收、分布、代谢和排泄 (ADME) 特性,并且具有口服生物利用度。 Dapagliflozin (1 mg/kg) 在正常大鼠给药后 24 小时内引起显着的剂量依赖性糖尿和尿量增加。 Dapagliflozin 会在 Zucker 糖尿病脂肪 (ZDF) 大鼠给药后 6 小时诱导尿糖和尿量排泄增加。即使治疗 2 周,达格列净仍可降低 ZDF 大鼠的空腹和餐后血糖水平,且没有任何肾或肝毒性标志。达格列净显着减少高血糖的发生,降低血糖。达格列净可以提高胰岛素敏感性,减少β细胞质量和胰腺功能受损的发展。
急性体内疗效。[2] 在正常大鼠中,达格列净给药引起明显的剂量依赖性糖尿(图1)和尿量增加,给药后24小时内,与赋形剂相比,1 mg/kg的尿糖增加了400倍,尿量增加了三倍。在正常大鼠的口服葡萄糖耐量试验中,在1和10mg/kg剂量下,达格列嗪给药与给药后1小时内曲线下葡萄糖面积的减少有关(图2),表明这种葡萄糖尿酸剂能够减少正常大鼠急性葡萄糖挑战后的葡萄糖波动。在单剂量口服达格列嗪的ZDF大鼠中,给药后6小时,尿糖和尿量排泄明显呈剂量依赖性增加(图3A),同时同一只大鼠在0.01-1.0 mg/kg的剂量下血糖降低(图4)。在24小时内检查时,尿糖排泄效应的明显剂量依赖性下降(图3B),所有剂量组的治疗大鼠的尿糖水平均比赋形剂治疗大鼠提高了两倍。在这些大鼠以1mg/kg的剂量重新进食一段时间后,在给药后24小时仍观察到降低血糖的疗效。在这些研究过程中没有观察到低血糖的证据。在1 mg/kg的剂量下,给药后1小时达格列净的血浆暴露量为1.2μmol/l,在实验的前6小时估计为5.2μmol·l-1·h-1。 慢性体内疗效。[2] 在两项慢性研究中的第一项中,Dapagliflozin剂量依赖性地降低了治疗第8天时禁食18小时的ZDF大鼠的空腹血糖水平,这是在前一次给药后24小时测量的(图5)。在治疗的第15天,大鼠禁食24小时,这种效果也很明显,在研究的第14天,喂食动物也有这种效果。这些数据表明,在为期2周的每日一次治疗方案中,降低FPG的疗效得以保持。与赋形剂处理的大鼠相比,没有发现体重变化,也没有观察到异常行为;老鼠似乎很好。没有测量到肾或肝毒性的标志物。[2] 在第二项慢性研究中,当在第15天最后一次给药后24小时进行测量时,与赋形剂治疗的大鼠相比,用0.5mg/kg dDapagliflozin治疗的ZDF大鼠在18小时的FPG水平下降了53%(表3)。在最后一次给药后的第三天,启动了一项高胰岛素血症正常血糖钳夹研究,以评估达格列嗪与赋形剂治疗的代谢影响。在基础阶段,与达格列嗪治疗的大鼠相比,赋形剂治疗的大白鼠的尿糖损失率明显更高(表3),这可能反映了赋形剂治疗大白鼠血浆葡萄糖水平升高的趋势。在大鼠最后一剂半衰期为4至5小时的化合物给药后48小时开始夹紧程序的事实(W.Humphreys,W.N.W.,未发表的数据)表明,夹紧程序期间的血浆药物水平可以忽略不计,尽管没有测量。因此,我们预计达格列嗪急性诱导的尿糖排泄对钳夹期间观察到的代谢效应没有显著影响。在此过程中,达格列嗪治疗的大鼠的尿量也显著减少。尽管与赋形剂处理的大鼠相比,达格列嗪治疗的大鼠在夹子的胰岛素输注阶段尿葡萄糖损失似乎有所减少,但这种差异在统计学上并不显著。[2] 与赋形剂处理的大鼠相比,在夹具的胰岛素输注阶段,达格列净处理的大白鼠的葡萄糖输注速率显著增加,表明全身葡萄糖利用率有所改善(表3)。与赋形剂处理的大鼠相比,达格列嗪治疗的大鼠在胰岛素输注阶段的葡萄糖产量也显著降低(表3)。此外,在达格列嗪治疗的大鼠中,钳夹期间肝脏对放射性标记葡萄糖的摄取显著增加,而骨骼肌或白色脂肪组织对葡萄糖的摄取没有显著变化。这些数据表明,用达格列净治疗2周可以改善ZDF大鼠葡萄糖产量的升高,并增强肝脏胰岛素敏感性。 从高脂肪喂养开始服用达格列净/Dapagliflozin,可以减少高血糖的发展;24天后,血糖为8.6±0.5 vs.13.3±1.3 mmol/l(与溶媒相比p<0.005),糖化血红蛋白为3.6±0.1 vs.4.8±0.26%(与赋形剂相比p<0.003)。达格列净改善了肥胖对照组的胰岛素敏感性指数:0.08±0.01比0.02±0.01(p<0.03)。DI改善到瘦对照组大鼠的水平(达格列净0.29±0.04;肥胖对照组0.15±0.01;瘦对照组0.28±0.01)。在达格列嗪治疗的大鼠中,β细胞质量的变化较小,与赋形剂治疗的大白鼠相比,胰岛形态有显著改善,尽管达格列嗪的平均β细胞质量没有变化。当动物已经中度高血糖时,开始达格列嗪治疗,结果相似。 结论:在2型糖尿病模型中,达格列净持续降糖可防止胰腺β细胞功能适应性的持续下降。[4] 成年大鼠在前3个月喂食富含果糖的饮食以诱导代谢综合征,然后用<Dapagliflozin达格列净 或含硫酸镁的饮用水治疗另外3个月。喂食果糖的动物胰岛素抵抗增加,低镁血症,尿镁排泄减少。达格列净治疗通过降低葡萄糖和胰岛素水平、增加血清镁水平和减少尿镁排泄来改善胰岛素抵抗。果糖喂养的动物血清维生素D和甲状旁腺激素水平降低,尽管补充了达格列嗪和镁,但水平仍然很低。在肾脏中,果糖喂养的动物的claudin-16、TRPM6/7和FXDY表达增加。达格列净增加了细胞内镁浓度,这种作用被TRPM6阻断剂和EGFR拮抗剂抑制。我们得出结论,高果糖摄入结合低镁饮食会导致代谢综合征和低镁血症。达格列嗪和硫酸镁补充剂都改善了代谢综合征的特征,并提高了血清镁水平。在果糖喂养的动物和服用达格列嗪和硫酸镁的动物中,claudin-16、TRPM6/7和FXYD2等镁转运蛋白的表达水平升高。达格列净增强肾小管细胞中TRPM6介导的跨上皮镁转运[5]。 |
| 酶活实验 |
选择性 SGLT 底物 α-甲基-D-吡喃葡萄糖苷 (AMG) 用于使用稳定表达人 SGLT2 (hSGLT2) 和人 SGLT1 (\hSGLT1) 的中国仓鼠卵巢 (CHO) 细胞开发转运测定。达格列净抑制 [14C]AMG 摄取的能力是在无蛋白质缓冲液中孵育两个小时的过程中测量的。通过将响应曲线拟合到经验四参数模型来找到半最大响应时的抑制剂浓度(或 EC50)。使用无蛋白缓冲液复制肾小球滤液的低蛋白环境,覆盖肾脏近端小管腔表面上的 SGLT 靶标。
SGLT1和SGLT2测定。[2] 使用标准细胞培养技术维持表达hSGLT1、hSGLT2、rSGLT1或rSGLT2的细胞。通过添加100μl/孔的含钠(HEPES/Tris pH 7.4、137 mmol/l NaCl、5.4 mmol/l KCl、2.8 mmol/l CaCl2、1.2 mmol/l MgSO4)、10μmol/l 14C-α-甲基-d-吡喃葡萄糖苷和抑制剂或DMSO载体的无蛋白测定缓冲液,启动96孔板中钠依赖性葡萄糖转运的测定,并将板在37°C下孵育2小时。钠依赖性14C-α-甲基-d-吡喃葡萄糖苷摄取量是通过从含钠条件下观察到的计数中减去无钠摄取条件下每分钟的计数来计算的。在钠存在的情况下,对八种浓度的抑制剂进行了三次检测,并通过比较含抑制剂的孔中每分钟的计数与仅含DMSO载体的孔中的每分钟计数来计算抑制百分比。在每次测定中平行评估Phlorizin。使用XL Fit将剂量反应曲线拟合到经验四参数模型中,以确定半最大反应时的抑制剂浓度(EC50)。 脂肪细胞葡萄糖摄取测定。[2] 在试验之前,将预分化的人脂肪细胞在Dulbecco改良的Eagle培养基中洗涤一次,该培养基为低葡萄糖,不含胎牛血清,并在37°C下孵育2小时。然后在不含葡萄糖的Krebs-Ringer碳酸氢盐HEPES缓冲液中洗涤细胞两次。测定缓冲液(100μl/孔)由不含胰岛素或100 nmol/l胰岛素的Krebs-Ringer碳酸氢盐HEPES缓冲液、10μmol/l 2-14C-脱氧-d-葡萄糖、抑制剂或细胞松弛素B以及DMSO对照(每组n=6)组成。细胞在37°C下孵育20分钟,在PBS中洗涤三次,并在50μl/孔的0.1 N NaOH中裂解。加入MicroScint-40,在TopCount闪烁计数器中计数细胞。通过比较含抑制剂孔中每分钟的计数与不含抑制剂孔的每分钟计数来计算抑制百分比。 |
| 细胞实验 |
在 30 分钟的缺血期间,使用载体或达格列净预处理 24 小时孵育期后,收集细胞用于细胞存活测定。然后使用台盼蓝染色对任何剩余的细胞进行计数。将处理的细胞的相对存活数除以未处理的细胞的存活数进行量化以获得存活百分比。
Caco-2通透性和P-gp相互作用。[3] 在pH 7.4、初始浓度为50μM时,达格列净在顶端(A)至基底外侧(B)方向的渗透系数为60 nm/s。该值与在人体中表现出中等吸收的化合物相当(Marino等人,2005)。在相同条件下,达格列嗪的平均B-to-A渗透系数值为227nm/s(BA/AB比为3.8)。在P-gp抑制剂存在的情况下,达格列嗪的a-to-B通透性为159 nm/s,B-to-a通透性为150 nm/s。。。 细胞培养和细胞内镁浓度测量。[5] 将NRK52E细胞接种在96孔荧光板上,并在以下条件下处理细胞24小时:1。常规培养基;2.10μM间充质;3.10μM AG1478;4.0.2μM达格列净;5.10μM间充质和0.2μM达格列嗪;6.10μM AG1478(0.2μM)和达格列嗪。然后将细胞与5μM Mg-Fura-2 AM在37°C下孵育60分钟,然后用所需的最终培养基洗涤三次。然后将细胞再孵育60分钟,以便在荧光测量之前完全脱除细胞内AM酯。所有实验重复4-6次,然后取平均值。 |
| 动物实验 |
Dissolved in 5% mpyrol, 20% PEG400, and 20 mM sodium diphosphate; 0.01-10 mg/kg (1 mL/kg) followed by a 50% glucose solution (2 g/kg); oral administration. Normal Sprague Dawley rats or streptozotocin induced male Sprague Dawley rats.
Diagrammatic representation of the protocols used in these experiments are available in an online appendix at http://dx.doi.org/10.2337/db07-1472. The animals were allowed ad libitum access to food and water unless otherwise stated, and rooms were maintained at 22°C and 50% humidity on a 12-h light/dark cycle. Male Sprague-Dawley rats were maintained on Harlan Teklad 2018 diet and weighed 250 g at the time of the experiment. Male Zucker diabetic fatty (ZDF) rats were maintained on Purina 5008 chow, and for acute studies, rats were 19 weeks of age and had a mean weight of 422 g. For the first chronic study, male ZDF rats were 17 weeks of age with a mean weight of 399 g; in the second chronic study, male ZDF rats were 15 weeks old with a mean body weight of 397 g. For all ZDF rat studies, rats were randomized into groups where body weight and plasma glucose levels were not statistically different between groups. Blood samples were collected and centrifuged (Eppendorf) at 4°C, 2,500 rpm, for 10 min. Plasma (5 μ1) was removed and mixed with 25 μl saline for glucose analysis using the Cobas Mira Analyzer. All urine volumes were measured and recorded. For urine glucose analysis, 5 μl urine was removed and mixed with 250 μl saline and analyzed on the Cobas Mira Analyzer. Acute normal and diabetic rat studies.[2] For all animal studies, the vehicle used for drug administration was 5% 1-methyl-2-pyrrolidinone, 20% polyethylene glycol, and 20 mmol/l sodium diphosphate. For glucose tolerance testing, 15 Sprague-Dawley rats were fasted overnight (18 h), weighed, bled via tail tip (30–40 μl), and randomized into five groups (n = 3). Rats were dosed orally with single doses of vehicle or drug (1 ml/kg; 0.01–10 mg/kg drug) and subsequently dosed orally with 50% aqueous glucose solution (2 g/kg). Rats were then bled at 15, 30, and 60 min and 24 h post-dose. Insulin was not measured in these studies. For glucosuria assessment, overnight-fasted Sprague-Dawley rats were placed into metabolism cages for baseline urine collection over 24 h. Rats were weighed, randomized into five groups (n = 3), dosed orally with single doses of vehicle or drug (1 ml/kg; 0.01–10 mg/kg drug), and subsequently dosed orally with 50% aqueous glucose solution (2 g/kg). Immediately after dosing, rats were returned to metabolism cages for 24-h urine collection and re-fed at 1 h after the glucose challenge. The delta area under the curve for plasma glucose from baseline glucose value was calculated using GraphPad Prism. The urine glucose and urine volume data were normalized per 200 g body weight. For assessment of acute glucosuria and plasma glucose effects in ZDF rats, the animals were weighed, bled via the tail tip (40–50 μl) in the fed state, and randomized into four groups (n = 6). Rats were dosed with vehicle or drug (1 ml/kg; 0.01–1.0 mg/kg drug) and placed into metabolism cages (without prior acclimation). Blood samples were collected immediately before dosing and at 2, 4, 6, and 24 h post-dose. Urine collections were obtained at 2, 4, 6, and 24 h post-dose. The animals were allowed to re-feed after the 6-h time point. Plasma samples at each time point were analyzed for the presence of glucose and Dapagliflozin. Urine glucose and urine volume data were normalized per 400 g body weight. Insulin was not measured in these studies. Chronic diabetic rat studies.[2] Two studies were performed in ZDF rats to evaluate the effects of multi-dose treatment with Dapagliflozin on prandial and fasting plasma glucose (FPG) and the metabolic profile of the rats after 2 weeks of treatment. In study 1, rats were fasted overnight, weighed, bled via the tail tip (40–50 μl), and randomized into four groups (n = 6 per group). Rats were dosed orally with vehicle or drug (1 ml/kg; 0.01–1.0 mg/kg drug) once daily for 14 days. Fasting body weight and plasma samples were obtained on days 8 (18-h fast) and 15 (24-h fast), and fed plasma samples were taken on day 14, 24 h after the previous dose. In study 2, ZDF rats were randomized into two groups (n = 6 per group) and dosed orally with vehicle or drug (1 ml/kg; 0.5 mg/kg drug) once daily for 15 days. Blood samples (40 μl) were obtained from 18-h–fasted rats from all groups by tail bleed on days 1, 8, and 15 of the study to determine plasma glucose levels. Neither fasting nor fed insulin was measured in these studies. A hyperinsulinemic-euglycemic clamp study was conducted with vehicle- and dapagliflozin-treated rats on day 17 and 48 h after the last dose of vehicle or dapagliflozin. Neither food nor water intake were monitored in these studies. In a preliminary study, four obese female ZDF rats were placed on C13004 high-fat diet for 12–15 days, while four matched obese and four lean animals remained on RM1 chow diet. A hyperglycaemic clamp study was then carried out as described in subsequent text. For the evaluation of Dapagliflozin, two separate studies were performed in parallel in two separate batches of rats. In the first study (‘prevention’), Dapagliflozin (n = 14) was administered from the initiation of high-fat feeding. In the second study (‘intervention’), dapagliflozin treatment (n = 14) was initiated 10 days after the start of high-fat diet when animals had become moderately hyperglycaemic. Dapagliflozin (1 mg/kg, p.o. in water) or vehicle was administered once daily at 08:00 h for 33 days. In both studies, 48 h after the final dose, following an overnight fast, all groups were subdivided into two matched subgroups based on day 24 glucose and glycated haemoglobin (gHb) levels. One subgroup (5–6 animals) was used for evaluation of pancreatic function by hyperglycaemic clamp. The remaining animals were rendered insentient by inhalation of a 5 : 1 mixture of CO2: O2 to minimize changes of glucose and insulin levels in blood samples taken by cardiac puncture into ethylenediaminetetraacetic acid (EDTA) blood syringes. The pancreas was removed and fixed in 10% neutral buffered formalin for 24–48 h, followed by conventional processing and embedding in paraffin wax.[4] Blood Sampling and Plasma Assays [4] Disease progression was monitored prestudy, and on days 14 and 24, by measurement of gHb and blood glucose from small samples taken from the tail vein in conscious animals. Samples were taken at 08:00 h prior to the dose of Dapagliflozin. Plasma insulin was measured by enzyme-linked immunosorbent assay (ELISA). Plasma C-peptide was measured by radioimmunoassay. The plasma triglyceride assay was carried out using a Roche modular system by the Glycero-3-phosphate oxidase - para aminophenazone method. Adult male Sprague Dawley rats weighing 200–250 g were used in this experiment. The animals were maintained under a constant 12 h photoperiod at temperatures between 21 °C and 23 °C. The animals were allowed free access to water and food. The animals were allocated to control and fructose-diet groups. Fructose diet groups were fed a fructose-rich diet (60% fructose, 0.05% magnesium wt/wt), and control animals (n = 10) received standard rat chow for 6 months. In animals receiving a high-fructose diet, drug treatments were started after 3 months of feeding. The animals were divided into three groups as follows: 1. continued fructose feeding for six months (FR, n = 10); 2. continued fructose diet with Dapagliflozin treatment (FR+Dapa, 1 mg·kg−1day−1 via oral gavage; n = 10) for 3 months; 3. continued fructose diet with magnesium sulfate supplementation (FR+Mg, 296 mg/L of magnesium in drinking water, Taiwan Biotech Co., Ltd., Taoyuan, Taiwan, n = 10) for 3 months. Blood pressure of each animal was measured by indirect tail cuff method twice a week. At least 5 readings were obtained and averaged. Body weight was measured weekly. At the end of the study, 24 h urine samples were collected in an individualized metabolic cage. The rats were then sacrificed, and blood samples were withdrawn from the inferior vena cava for biochemical analysis. [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral dapagliflozin reaches a maximum concentration within 1 hour of administration when patients have been fasting. Following oral administration of dapagliflozin, the maximum plasma concentration (Cmax) is usually attained within 2 hours under fasting state. The Cmax and AUC values increase dose proportionally with an increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10 mg dose is 78%. Administration of dapagliflozin with a high-fat meal decreases its Cmax by up to 50% and prolongs Tmax by approximately 1 hour but does not alter AUC as compared with the fasted state. These changes are not considered to be clinically meaningful and dapagliflozin can be administered with or without food. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway. Following a single 50 mg dose of [14C]-dapagliflozin, 75% and 21% of total radioactivity is excreted in urine and feces, respectively. In urine, less than 2% of the dose is excreted as the parent drug. In feces, approximately 15% of the dose is excreted as the parent drug. The volume of distribution was estimated to be 118L. Oral plasma clearance was 4.9 mL/min/kg, and renal clearance was 5.6 mL/min. Metabolism / Metabolites Dapagliflozin is primarily glucuronidated to become the inactive 3-O-glucuronide metabolite(60.7%). Dapagliflozin also produces another minor glucuronidated metabolite(5.4%), a de-ethylated metabolite(<5%), and a hydroxylated metabolite(<5%). Metabolism of dapagliflozin is mediated by cytochrome p-450(CYP)1A1, CYP1A2, CYP2A6, CYP2C9, CYP2D6, CYP3A4, uridine diphosphate glucuronyltransferase(UGT)1A9, UGT2B4, and UGT2B7. Glucuronidation to the major metabolite is mediated by UGT1A9. Biological Half-Life The mean plasma terminal half-life (t1/2) for dapagliflozin is approximately 12.9 hours following a single oral dose of 10 mg. In healthy subjects given a single oral dose of 50 mg of dapagliflozin, the mean terminal half-life was 13.8 hours. (2S,3R,4R,5S,6R)-2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6-hydroxymethyl-tetrahydro-2H-pyran-3,4,5-triol (dapagliflozin; BMS-512148) is a potent sodium-glucose cotransporter type II inhibitor in animals and humans and is currently under development for the treatment of type 2 diabetes. The preclinical characterization of dapagliflozin, to allow compound selection and prediction of pharmacological and dispositional behavior in the clinic, involved Caco-2 cell permeability studies, cytochrome P450 (P450) inhibition and induction studies, P450 reaction phenotyping, metabolite identification in hepatocytes, and pharmacokinetics in rats, dogs, and monkeys. Dapagliflozin was found to have good permeability across Caco-2 cell membranes. It was found to be a substrate for P-glycoprotein (P-gp) but not a significant P-gp inhibitor. Dapagliflozin was not found to be an inhibitor or an inducer of human P450 enzymes. The in vitro metabolic profiles of dapagliflozin after incubation with hepatocytes from mice, rats, dogs, monkeys, and humans were qualitatively similar. Rat hepatocyte incubations showed the highest turnover, and dapagliflozin was most stable in human hepatocytes. Prominent in vitro metabolic pathways observed were glucuronidation, hydroxylation, and O-deethylation. Pharmacokinetic parameters for dapagliflozin in preclinical species revealed a compound with adequate oral exposure, clearance, and elimination half-life, consistent with the potential for single daily dosing in humans. The pharmacokinetics in humans after a single dose of 50 mg of [(14)C]dapagliflozin showed good exposure, low clearance, adequate half-life, and no metabolites with significant pharmacological activity or toxicological concern. [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of dapagliflozin during breastfeeding. Dapagliflozin is an uncharged molecule that is 91% protein bound in plasma, so it is unlikely to pass into breastmilk in clinically important amounts. The manufacturer does not recommend dapagliflozin during breastfeeding because of a theoretical risk to the infant's developing kidney. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Dapagliflozin is approximately 91% protein bound. Protein binding is not altered in patients with renal or hepatic impairment. |
| 参考文献 |
|
| 其他信息 |
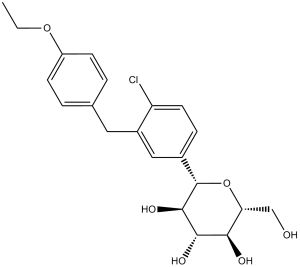
Dapagliflozin is a C-glycosyl comprising beta-D-glucose in which the anomeric hydroxy group is replaced by a 4-chloro-3-(4-ethoxybenzyl)phenyl group. Used (in the form of its propanediol monohydrate) to improve glycemic control, along with diet and exercise, in adults with type 2 diabetes. It has a role as a hypoglycemic agent and a sodium-glucose transport protein subtype 2 inhibitor. It is a C-glycosyl compound, an aromatic ether and a member of monochlorobenzenes.
Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and it was the first SLGT2 inhibitor to be approved. indicated for managing diabetes mellitus type 2. When combined with diet and exercise in adults, dapagliflozin helps to improve glycemic control by inhibiting glucose reabsorption in the proximal tubule of the nephron and causing glycosuria. Dapagliflozin has been investigated either as monotherapy or as an adjunct treatment with insulin or other oral hypoglycemic agents. Dapagliflozin was originally approved by the FDA on Jan 08, 2014, to improve glycemic control in adults with type 2 diabetes in conjunction with diet and exercise. It was later approved to reduce the risk of kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adults with chronic kidney disease in April 2021. Dapagliflozin is a Sodium-Glucose Cotransporter 2 Inhibitor. The mechanism of action of dapagliflozin is as a Sodium-Glucose Transporter 2 Inhibitor. Dapagliflozin is a selective sodium-glucose co-transporter subtype 2 (SGLT2) inhibitor with antihyperglycemic activity. Dapagliflozin selectively and potently inhibits SGLT2 compared to SGLT1, which is the cotransporter of glucose in the gut. DAPAGLIFLOZIN is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2012 and has 3 approved and 37 investigational indications. Pharmacodynamics Dapagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to, lowering both pre- and afterload of the heart and downregulation of sympathetic activity, and decreased intraglomerular pressure which is believed to be mediated by increased tubuloglomerular feedback. Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following the administration of dapagliflozin. Dapagliflozin doses of 5 or 10 mg per day in patients with type 2 diabetes mellitus for 12 weeks resulted in excretion of approximately 70 grams of glucose in the urine per day at Week 12. A near-maximum glucose excretion was observed at the dapagliflozin daily dose of 20 mg. This urinary glucose excretion with dapagliflozin also results in increases in urinary volume. After discontinuation of dapagliflozin, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days for the 10 mg dose. Dapagliflozin was not associated with clinically meaningful prolongation of QTc interval at daily doses up to 150 mg (15 times the recommended maximum dose) in a study of healthy subjects. In addition, no clinically meaningful effect on QTc interval was observed following single doses of up to 500 mg (50 times the recommended maximum dose) of dapagliflozin in healthy subjects. The C-aryl glucoside 6 (dapagliflozin) was identified as a potent and selective hSGLT2 inhibitor which reduced blood glucose levels in a dose-dependent manner by as much as 55% in hyperglycemic streptozotocin (STZ) rats. These findings, combined with a favorable ADME profile, have prompted clinical evaluation of dapagliflozin for the treatment of type 2 diabetes.[1] Objective: The inhibition of gut and renal sodium-glucose cotransporters (SGLTs) has been proposed as a novel therapeutic approach to the treatment of diabetes. We have identified dapagliflozin as a potent and selective inhibitor of the renal sodium-glucose cotransporter SGLT2 in vitro and characterized its in vitro and in vivo pharmacology. Research design and methods: Cell-based assays measuring glucose analog uptake were used to assess dapagliflozin's ability to inhibit sodium-dependent and facilitative glucose transport activity. Acute and multi-dose studies in normal and diabetic rats were performed to assess the ability of dapagliflozin to improve fed and fasting plasma glucose levels. A hyperinsulinemic-euglycemic clamp study was performed to assess the ability of dapagliflozin to improve glucose utilization after multi-dose treatment. Results: Dapagliflozin potently and selectively inhibited human SGLT2 versus human SGLT1, the major cotransporter of glucose in the gut, and did not significantly inhibit facilitative glucose transport in human adipocytes. In vivo, dapagliflozin acutely induced renal glucose excretion in normal and diabetic rats, improved glucose tolerance in normal rats, and reduced hyperglycemia in Zucker diabetic fatty (ZDF) rats after single oral doses ranging from 0.1 to 1.0 mg/kg. Once-daily dapagliflozin treatment over 2 weeks significantly lowered fasting and fed glucose levels at doses ranging from 0.1 to 1.0 mg/kg and resulted in a significant increase in glucose utilization rate accompanied by a significant reduction in glucose production. Conclusions: These data suggest that dapagliflozin has the potential to be an efficacious treatment for type 2 diabetes. [2] Aims: To investigate whether glucose lowering with the selective sodium glucose transporter 2 (SGLT2) inhibitor dapagliflozin would prevent or reduce the decline of pancreatic function and disruption of normal islet morphology. Methods: Female Zucker diabetic fatty (ZDF) rats, 7-8 weeks old, were placed on high-fat diet. Dapagliflozin (1 mg/kg/day, p.o.) was administered for ∼33 days either from initiation of high-fat diet or when rats were moderately hyperglycaemic. Insulin sensitivity and pancreatic function were evaluated using a hyperglycaemic clamp in anaesthetized animals (n = 5-6); β-cell function was quantified using the disposition index (DI) to account for insulin resistance compensation. Pancreata from a matched subgroup (n = 7-8) were fixed and β-cell mass and islet morphology investigated using immunohistochemical methods.[4] The prevalence of metabolic syndrome (MetS) is increasing, and patients with MetS are at an increased risk of cardiovascular disease and diabetes. There is a close link between hypomagnesemia and MetS. Administration of sodium-glucose transporter 2 (SGLT2) inhibitors has been reported to increase serum magnesium levels in patients with diabetes. We investigated the alterations in renal magnesium handling in an animal model of MetS and analyzed the effects of SGLT2 inhibitors. Adult rats were fed a fructose-rich diet to induce MetS in the first 3 months and were then treated with either dapagliflozin or magnesium sulfate-containing drinking water for another 3 months. Fructose-fed animals had increased insulin resistance, hypomagnesemia, and decreased urinary magnesium excretion. Dapagliflozin treatment improved insulin resistance by decreasing glucose and insulin levels, increased serum magnesium levels, and reduced urinary magnesium excretion. Serum vitamin D and parathyroid hormone levels were decreased in fructose-fed animals, and the levels remained low despite dapagliflozin and magnesium supplementation. In the kidney, claudin-16, TRPM6/7, and FXDY expression was increased in fructose-fed animals. Dapagliflozin increased intracellular magnesium concentration, and this effect was inhibited by TRPM6 blockade and the EGFR antagonist. We concluded that high fructose intake combined with a low-magnesium diet induced MetS and hypomagnesemia. Both dapagliflozin and magnesium sulfate supplementation improved the features of MetS and increased serum magnesium levels. Expression levels of magnesium transporters such as claudin-16, TRPM6/7, and FXYD2 were increased in fructose-fed animals and in those administered dapagliflozin and magnesium sulfate. Dapagliflozin enhances TRPM6-mediated trans-epithelial magnesium transport in renal tubule cells.[5] |
| 分子式 |
C21H25CLO6
|
|---|---|
| 分子量 |
408.87
|
| 精确质量 |
408.133
|
| 元素分析 |
C, 61.69; H, 6.16; Cl, 8.67; O, 23.48
|
| CAS号 |
461432-26-8
|
| 相关CAS号 |
Dapagliflozin ((2S)-1,2-propanediol, hydrate); 960404-48-2; Dapagliflozin-d5; 1204219-80-6
|
| PubChem CID |
9887712
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
609.0±55.0 °C at 760 mmHg
|
| 闪点 |
322.1±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.614
|
| LogP |
4.42
|
| tPSA |
99.38
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
472
|
| 定义原子立体中心数目 |
5
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])C([H])([H])[H])[C@@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])O[H])O[H]
|
| InChi Key |
JVHXJTBJCFBINQ-ADAARDCZSA-N
|
| InChi Code |
InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1
|
| 化学名 |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
|
| 别名 |
Dapagliflozin; BMS 512148; BMS512148; Dapagliflozin; 461432-26-8; BMS-512,148; Forxiga; Farxiga; BMS 512,148; (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; dapagliflozine; BMS-512148; trade name Farxiga in the US and Forxiga in the EU
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.11 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.11 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (5.09 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (5.09 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: ≥ 0.5 mg/mL (1.22 mM) (饱和度未知) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 7 中的溶解度: 30% PEG400+0.5% Tween80+5% Propylene glycol : 30 mg/mL 配方 8 中的溶解度: 33.33 mg/mL (81.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4458 mL | 12.2288 mL | 24.4577 mL | |
| 5 mM | 0.4892 mL | 2.4458 mL | 4.8915 mL | |
| 10 mM | 0.2446 mL | 1.2229 mL | 2.4458 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Vitamin D and SGLT-2 Inhibitor in CPAP-naive Obstructive Sleep Apnea
CTID: NCT06690723
Phase: Phase 3 Status: Completed
Date: 2024-11-15
|
|
|
|