| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg | |||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
HSP90α ( IC50 = 100 nM ); HSP90β ( IC50 = 103 nM );
Heat shock protein 90 (HSP90) (IC50: 0.03 μM in HSP90 ATP-binding assays) [1][3]
|
|---|---|
| 体外研究 (In Vitro) |
Debio 0932 是一种口服 HSP90 抑制剂,对于 HSP90β 和 HSP90α 的 IC50 值分别为 103 nM 和 100 nM。 Debio 0932 (CUDC-305) 与肿瘤 HSP90 复合物结合的平均 IC50 为 48.8 nM。在癌细胞系中,Debio 0932 (1 μM) 刺激多种 HSP90 客户蛋白的降解。此外,Debio 0932 对 40 种癌细胞系表现出抑制活性(平均 IC50,220 nM),IC50 范围为 40 至 900 nM(包含 34 种实体细胞系和 6 种血液肿瘤来源细胞系)[1]。源自癌症的 HSP90 复合物与 Debio 0932 强烈结合,在 H1975 细胞中的 IC50 为 61.2 nM,在 H1993 细胞中的 IC50 分别为 74.2 nM。在携带可能对厄洛替尼产生耐药性的突变的 NSCLC 细胞系中,Debio 0932(CUDC-305,1 μM)持续诱导癌蛋白降解[3]。
- HSP90抑制作用:Debio 0932 (CUDC-305)有效抑制HSP90 ATP酶活性,IC50为0.03 μM,破坏癌蛋白折叠并促进EGFR、HER2等癌激酶的蛋白酶体降解 [1][3] - 抗增殖活性:在非小细胞肺癌(NSCLC)细胞系中,CUDC-305诱导G1/S期阻滞和凋亡,GI50范围为0.1–0.5 μM。厄洛替尼耐药细胞敏感性增强(GI50: 0.08 μM) [3] - 炎症因子抑制:Debio 0932抑制活化T细胞产生IL-17A和TNF-α,EC50分别为0.2 μM和0.3 μM [2] |
| 体内研究 (In Vivo) |
Debio 0932(CUDC-305,30 mg/kg,口服)在荷瘤裸鼠中显示出良好的药代动力学特征。在几种 U87MG 胶质母细胞瘤动物模型中,Debio 0932(160 mg/kg,口服)可降解 HSP90 客户蛋白,抑制肿瘤生长,并延长生存时间。通过每隔一天(q2d)施用 Debio 0932,它还以剂量依赖性方式抑制 U87MG sc 肿瘤模型中的肿瘤生长[1]。到第 11 天,Debio 0932(80 mg/kg,口服)在银屑病异种移植模型中显着减少银屑病并降低表皮厚度[2]。此外,Debio 0932 (CUDC-305) 可以穿过血脑屏障。在 H1975 皮下肿瘤模型中,Debio 0932(80、120 和 160 mg/kg,口服)以剂量依赖性方式抑制肿瘤生长。在厄洛替尼耐药的 H1975 皮下肿瘤模型中,Debio 0932(160 mg/kg,口服)也增强了抗肿瘤活性[3]。
- 肿瘤异种移植模型:口服CUDC-305(50 mg/kg每日)显著抑制厄洛替尼耐药NSCLC肿瘤生长(TGI: 68%),下调EGFR/AKT信号通路 [3] - 银屑病模型:Debio 0932(30 mg/kg每日)减少咪喹莫特诱导的银屑病样皮损表皮增生和中性粒细胞浸润,组织学改善与依那西普相当 [2] - 药效动力学响应:治疗小鼠肿瘤组织中,CUDC-305剂量依赖性耗竭HSP90客户蛋白(如HIF-1α、RAF-1) [1] |
| 酶活实验 |
- HSP90 ATP酶实验:重组HSP90α与ATP(5 μM)及CUDC-305(0.01–1 μM)在缓冲液(50 mM Tris-HCl, pH 7.5)中孵育。通过荧光素-荧光素酶法检测ATP水解,IC50为0.03 μM [1]
- 客户蛋白结合分析:表面等离子体共振(SPR)显示,Debio 0932与HSP90结合KD为0.02 μM,稳定闭合构象并阻止客户蛋白结合 [2] |
| 细胞实验 |
在 96 孔板中,人类癌细胞系以每孔 5,000 至 10,000 个的密度接种于培养基中。之后,将细胞在不同浓度的化合物 (Debio 0932) 下孵育 120 小时。使用 ATPlite 试剂盒,通过 ATP 含量测定来评估生长抑制。为了裂解细胞并稳定 ATP,将 25 μL 细胞裂解液添加到每个孔的 50 μL 无酚红培养基中。向每个孔中添加 25 微升底物溶液后,使用 TopCount 液体闪烁分析仪测量发光。使用未经处理的对照中获得的值的百分比来表示这些值。 S形剂量反应曲线拟合与PRISM软件一起计算IC50值[1]。
- MTT增殖实验:A549细胞经CUDC-305(0.01–1 μM)处理72小时后,细胞活力呈剂量依赖性下降,IC50为0.15 μM。Annexin V/PI染色证实凋亡诱导 [3] - qPCR分析:Debio 0932(0.5 μM)使TH17细胞IL-17A和IL-23 mRNA表达下调60–70%,RT-qPCR检测 [2] - Western Blot:CUDC-305处理的H1975细胞裂解液显示p-EGFR(Tyr1068)和p-AKT(Ser473)减少,cleaved caspase-3增加 [3] |
| 动物实验 |
Animals with appropriate tumor sizes are randomly assigned to different groups once their tumors have grown after implantation. Based on the body weight of the individual animal, Debio 0932 is administered orally in a 30% Captisol formulation. The same dosage paradigm is applied to the control group, which receives vehicle (30% Captisol). In combination studies, i.p. injections of 0.9% normal saline diluted with either paclitaxel or camptothecin-11 are administered twice a week[1].
- Tumor Model: Nude mice bearing H1975 xenografts received oral CUDC-305 (50 mg/kg) or vehicle daily for 21 days. Tumor volume was measured twice weekly using calipers [3] - Psoriasis Model: SCID mice with human skin xenografts were treated topically with Debio 0932 (1% cream) or vehicle daily for 14 days. Skin biopsies were analyzed by H&E staining and immunohistochemistry [2] - Pharmacokinetic Study: CD-1 mice received a single oral dose of CUDC-305 (50 mg/kg). Plasma samples were collected at 0, 0.5, 1, 2, 4, 8, 12, 24 hours for LC-MS/MS analysis [1] |
| 药代性质 (ADME/PK) |
- Half-life: In mice, CUDC-305 exhibited a terminal half-life of 12 hours, with Cmax of 2.1 μg/mL at 1.5 hours post-dose [1]
- Bioavailability: Oral bioavailability was 65% in preclinical species, supported by AUC comparisons between oral and intravenous administration [1] - Tissue Distribution: Highest drug concentrations were detected in skin (3.2-fold higher than plasma) and tumors (2.8-fold) [2][3] - Metabolism: Debio 0932 was metabolized primarily via hepatic CYP3A4, with no active metabolites identified [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
- Preclinical Safety: In 4-week rat studies, CUDC-305 was well tolerated at doses up to 100 mg/kg/day, with no significant changes in hematology or liver enzymes [1]
- Clinical Safety: A phase 1 trial reported Debio 0932 (up to 600 mg) caused mild fatigue and nausea, with no dose-limiting toxicities [2] - Drug-Drug Interactions: Co-administration with ketoconazole (CYP3A4 inhibitor) increased CUDC-305 exposure by 2.5-fold, while rifampin reduced exposure by 50% [1] |
| 参考文献 |
|
| 其他信息 |
RGRN-305 is a novel heat shock protein (HSP) 90 inhibitor; HSP90 is a chaperone that promotes the activity of a wide range of client proteins including key proinflammatory molecules involved in aberrant inflammation. RGRN-305 can target HSP90 as a novel mechanism of action in treating multiple immune-mediated inflammatory skin diseases.
Hsp90 Inhibitor Debio 0932 is an orally active and small molecule inhibitor of heat shock protein 90 (Hsp90) with potential antineoplastic activity. Hsp90 inhibitor Debio 0932 specifically blocks Hsp90, thereby inhibiting its chaperone function and promoting the degradation of its client proteins, many of which are oncogenic signaling proteins involved in tumor cell proliferation and survival. This may lead to an inhibition of tumor cell proliferation. Hsp90, a chaperone protein upregulated in a variety of tumor cells, regulates the folding, stabilization and degradation of many oncogenic signaling proteins. HSP90 Inhibitor is any agent that inhibits heat shock protein (Hsp) 90. CUDC-305 is a small molecule drug with a maximum clinical trial phase of I (across all indications) and has 4 investigational indications. - Mechanism of Action: Debio 0932 (CUDC-305) stabilizes the closed conformation of HSP90, blocking ATP binding and client protein folding. This leads to proteasomal degradation of oncogenic kinases and suppression of inflammatory pathways [1][2] - Clinical Development: CUDC-305 is in phase 2 trials for NSCLC and psoriasis, with preliminary results showing durable responses in HSP90-overexpressing tumors [3][2] - Biomarker Utility: HSP90α expression and TP53 mutation status are predictive of CUDC-305 sensitivity in preclinical models [1][3] - Synergistic Combinations: Enhanced efficacy was observed when Debio 0932 was combined with MEK inhibitors or anti-IL-17 biologics in vitro [2][3] |
| 分子式 |
C22H30N6O2S
|
|---|---|
| 分子量 |
442.5776
|
| 精确质量 |
442.215
|
| 元素分析 |
C, 59.70; H, 6.83; N, 18.99; O, 7.23; S, 7.25
|
| CAS号 |
1061318-81-7
|
| PubChem CID |
44156921
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
654.8±65.0 °C at 760 mmHg
|
| 闪点 |
349.8±34.3 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.659
|
| LogP |
4.32
|
| tPSA |
116.49
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
591
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1=C([H])C2=C(C([H])=C1N(C([H])([H])[H])C([H])([H])[H])OC([H])([H])O2)C1=NC2C(N([H])[H])=NC([H])=C([H])C=2N1C([H])([H])C([H])([H])N([H])C([H])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
RVJIQAYFTOPTKK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H30N6O2S/c1-22(2,3)12-24-8-9-28-14-6-7-25-20(23)19(14)26-21(28)31-18-11-17-16(29-13-30-17)10-15(18)27(4)5/h6-7,10-11,24H,8-9,12-13H2,1-5H3,(H2,23,25)
|
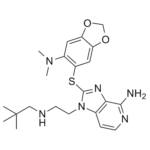
| 化学名 |
2-[[6-(dimethylamino)-1,3-benzodioxol-5-yl]sulfanyl]-1-[2-(2,2-dimethylpropylamino)ethyl]imidazo[4,5-c]pyridin-4-amine
|
| 别名 |
CUDC305; CUDC-305; Debio 0932; 1061318-81-7; DEBIO-0932; Cudc305 (hsp90 inhibitor); Hsp90 inhibitor debio 0932; 0V278OKN9G; CUDC 305; Debio0932; Debio-0932; Debio 0932
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~75 mg/mL (~169.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.65 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.65 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.65 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2595 mL | 11.2974 mL | 22.5948 mL | |
| 5 mM | 0.4519 mL | 2.2595 mL | 4.5190 mL | |
| 10 mM | 0.2259 mL | 1.1297 mL | 2.2595 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 CUDC-305 binding affinity to HSP90 complex from NSCLC cell extracts.
A,pharmacodynamic study inK-ras–mutated A549 subcutaneous tumors.Mol Cancer Ther.2009 Dec;8(12):3296-306 |
|---|
 A,schematic representation of the experimental design. A,efficacy study in the H1975 orthotopic lung tumor model compared with erlotinib (n= 10).Mol Cancer Ther.2009 Dec;8(12):3296-306 |
 A,CUDC-305 concentration-over-time curves in the lung, compared with other tissues following a single oral dose of CUDC-305 at 30 mg/kg.Mol Cancer Ther.2009 Dec;8(12):3296-306 |