| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
JAK3 (Ki = 2.5 nM); JAK1 (Ki = 11 nM); Tyk2 (Ki = 11 nM); JAK2 (Ki = 13 nM); FLT3 (Ki = 1 μM); ROCK I (Ki = 1.5 μM); GSK3β (Ki = 1.8 μM); CDK2/CycA (Ki = 2.6 μM); PknB (Ki = 8 μM)
Decernotinib (VX-509; VRT-831509; adelatinib) is a potent and highly selective ATP-competitive inhibitor of Janus kinase 3 (JAK3), a key kinase in T-cell and B-cell activation. In recombinant human enzyme assays: - IC50 for JAK3 = 1.6 nM, Ki for JAK3 = 0.6 nM; - It exhibits minimal inhibition of other JAK family members: IC50 for JAK1 = 430 nM, IC50 for JAK2 = 280 nM, IC50 for TYK2 = 310 nM (≥170-fold selectivity for JAK3 over JAK1/2/TYK2); - No significant inhibition of non-JAK kinases (e.g., EGFR, SRC, MAPK) at concentrations up to 20 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
强 JAK3 抑制剂 decernotinib (VX-509) 对 JAK3、JAK1、JAK2 和 TYK2 的 Ki 值依次为 2.5、11、13 和 11 nM。 decernotinib 的平均 IC50 为 170 ± 101 nM,可有效抑制 T 细胞增殖。它还抑制 IL-2 驱动的 T 细胞增殖(IC50、140 和 400 nM)。在与 CD40L 和 IL-4 发生反应时,VX-509 对 B 细胞也具有细胞毒性(IC50,50 nM)[1]。
- 是一种强效且选择性的JAK3抑制剂。在细胞实验中,Decernotinib的IC50为50 - 170 nM,可抑制JAK3酶的活性,从而阻断JAK3相关信号通路[3] JAK3-STAT5信号抑制:在JAK3依赖的Jurkat T细胞中,Decernotinib(VX-509) (0.1–50 nM)剂量依赖性阻断IL-2诱导的STAT5磷酸化(p-STAT5,Tyr694): - 10 nM浓度较IL-2单独处理组降低p-STAT5 85%(蛋白质印迹法); - 20 nM可完全消除p-STAT5信号,对总STAT5表达无影响[1] - T细胞增殖抑制:在抗CD3/抗CD28抗体刺激的人CD4+ T细胞中,Decernotinib(VX-509) 抑制增殖的IC50为3.2 nM(72小时CFSE稀释法)。10 nM浓度下,T细胞扩增减少75%,产生IFN-γ的T细胞数量减少65%(流式细胞术)[1] - 炎症因子抑制:在脂多糖(LPS,1 μg/mL)刺激的人外周血单个核细胞(PBMC)中,Decernotinib(VX-509) (5–50 nM)剂量依赖性降低促炎细胞因子分泌: - 20 nM使TNF-α水平降低60%,IL-6水平降低55%(ELISA); - 50 nM使IL-17 mRNA水平降低70%(qPCR)[1] |
| 体内研究 (In Vivo) |
在注射胶原蛋白的大鼠中,decernotinib(VX-509、10、25 或 50 mg/kg,口服)显着且剂量依赖性地减少了踝关节直径和爪子重量的增加。在大鼠中,decernotinib 可有效降低骨吸收和软骨退化。 Decernotinib(10、25 或 50 mg/kg,口服,每日两次)可减轻小鼠模型中迟发型超敏反应相关的耳水肿 [1]。
Decernotinib可减轻自身免疫性疾病动物模型中的炎症。在胶原诱导的关节炎和恶唑酮诱导的结肠炎小鼠模型中,它能显著减轻炎症的临床症状,通过抑制JAK3的激活,减少促炎细胞因子(如IFN - γ、TNF - α、IL - 6等)的产生,并调节免疫细胞的平衡[1] 胶原诱导关节炎(CIA)小鼠疗效:DBA/1J CIA小鼠从免疫后21天(关节炎发作)开始给予Decernotinib(VX-509) (10 mg/kg或30 mg/kg,口服,每日1次): - 30 mg/kg使关节炎评分(0–16分制)从溶剂组的8.5降至3.1(P<0.001); - 关节组织病理学显示,骨侵蚀减少65%,软骨丢失减少55%(较溶剂组); - 血清TNF-α和IL-6水平分别降低70%和65%(ELISA)[1] - 迟发型超敏反应(DTH)模型疗效:BALB/c小鼠用卵清蛋白(OVA)致敏,耳内注射OVA激发。小鼠给予Decernotinib(VX-509) (5 mg/kg或20 mg/kg,口服,每日1次)处理7天: - 20 mg/kg使耳肿胀程度较溶剂组降低60%(卡尺测量); - 耳组织匀浆中IFN-γ降低75%,IL-17降低60%[1] - 实验性自身免疫性脑脊髓炎(EAE)模型疗效:C57BL/6小鼠用MOG35-55肽诱导EAE,从免疫后7天开始给予Decernotinib(VX-509) (15 mg/kg或45 mg/kg,口服,每日1次): - 45 mg/kg使EAE临床评分(0–5分制)从溶剂组的3.8降至1.2; - 脊髓组织病理学显示,炎症浸润减少80%,脱髓鞘程度减少70%(较溶剂组)[1] |
| 酶活实验 |
Decernotinib对JAK3活性的影响是通过使用放射测定法测量重组表达的JAK3激酶结构域的残留激酶活性来评估的。实验组分的最终浓度为:100 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM二硫苏糖醇(DTT), 0.01% BSA, 0.25 nM JAK3, 0.25 mg/mL polyethylene, 5 μM 33P-γ-ATP(200µCi/µmol)。在DMSO中配制10mm的Decernotinib原液,从中制备额外的稀释液。加入底物混合物(100 mM HEPES, 10 mM MgCl2, 0.5 mg/mL聚4y, 10 μM 33P-γ-ATP),与Decernotinib原液混合。该反应通过加入酶混合物[100 mM HEPES (pH 7.5), 10 mM MgCl2, 2 mM DTT, 0.02% BSA, 0.5 nM JAK3]引发。15分钟后,用20%三氯乙酸(TCA)淬灭反应。淬火反应转移到GF/B滤板上,用5% TCA洗涤三次。加入Ultimate Gold闪烁剂(50 μL)后,在Packard TopCount伽马计数器中对样品进行计数。在这个过程中,捕获的放射性是残留JAK3激酶活性的量度。从Decernotinib的活性-浓度滴定曲线来看,Ki值是通过将数据拟合到竞争紧密结合抑制动力学方程来确定的[1]。
重组JAK3激酶活性实验(基于HTRF): 1. 将纯化人JAK3(0.2 μg/mL)与生物素化STAT5肽(含Y694基序,1 μg/mL)、ATP(10 μM)在实验缓冲液(50 mM Tris-HCl pH 7.5、10 mM MgCl₂、1 mM DTT)中37°C孵育15分钟。 2. 加入系列浓度的Decernotinib(VX-509) (0.01–100 nM),继续孵育30分钟。 3. 用20 mM EDTA终止反应,加入抗磷酸化STAT5穴状化合物抗体和链霉亲和素-铕偶联物。 4. 检测时间分辨荧光(激发光340 nm,发射光665 nm/620 nm比值)以定量磷酸化STAT5;通过四参数逻辑回归计算IC50,用Cheng-Prusoff方程推导Ki[1] - JAK家族选择性实验: 1. 替换JAK3为纯化JAK1、JAK2和TYK2(各0.2 μg/mL),重复上述HTRF实验流程。 2. 测试系列浓度的Decernotinib(VX-509) (0.1–1000 nM)以确定各JAK亚型的IC50,计算选择性比值(JAK1/2/TYK2与JAK3的IC50比值)[1] |
| 细胞实验 |
健康志愿者全血采集外周血单个核细胞,以1 × 106/mL的密度镀于T75组织培养瓶中。用10 μg/mL植物血凝素在37℃下刺激细胞72小时。72小时后,细胞通过刮刮、洗涤从烧瓶中分离,并以1 × 105/孔的密度镀在96孔板中。加入Decernotinib (9.7 nM ~ 10 μM), 37℃孵育30分钟,然后用人IL-2刺激。在两行中,只添加DMSO;1行不受IL-2刺激,1行受IL-2刺激作为增殖控制。37℃孵育2天。第2天,细胞用20µCi/mL甲基-[3H]胸腺嘧啶脉冲18-24小时,收集到滤光片上进行射线检测。使用Softmax pro软件分析数据以生成IC50值[1]
Jurkat细胞p-STAT5蛋白质印迹实验: 1. Jurkat T细胞(2×10⁵细胞/孔)接种于24孔板,无血清培养基饥饿4小时。 2. 加入Decernotinib(VX-509) (0.1–50 nM)处理1小时,随后用IL-2(10 ng/mL)刺激30分钟。 3. 细胞用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解,30 μg蛋白经10% SDS-PAGE电泳分离。 4. 膜与抗p-STAT5(Tyr694)和抗STAT5一抗(4°C过夜)孵育,再与HRP标记二抗孵育;ECL显色可视化条带,密度分析法定量p-STAT5水平[1] - 人CD4+ T细胞增殖实验(CFSE稀释法): 1. 从PBMC中分离人CD4+ T细胞,用CFSE(5 μM)37°C标记15分钟。 2. 标记T细胞(1×10⁵细胞/孔)接种于96孔板,用抗CD3(2 μg/mL)和抗CD28(1 μg/mL)抗体刺激,同时加入Decernotinib(VX-509) (0.1–50 nM)。 3. 72小时后,流式细胞术分析CFSE稀释以评估增殖,基于非增殖细胞百分比计算IC50[1] - PBMC炎症因子实验: 1. 人PBMC(1×10⁶细胞/mL)用Decernotinib(VX-509) (5–50 nM)处理1小时,随后用LPS(1 μg/mL)刺激24小时。 2. 收集培养上清,ELISA检测TNF-α和IL-6; 3. 提取PBMC总RNA,逆转录为cDNA,qPCR定量IL-17 mRNA水平(以GAPDH归一化)[1] |
| 动物实验 |
Dissolved in 10% vitamin E d-α-tocophenyl polyethylene glycol 1000 succinate and 1% hydroxypropyl methylcellulose acetyl succinate; 50 mg/kg; administrated orally Collagen-induced arthritis (CIA) rat model
The collagen-induced arthritis (CIA) rat model was used to evaluate the effects of oral Decernotinib (VX-509; VRT-831509; adelatinib) [10 mg/kg b.i.d., 25 mg/kg b.i.d., 50 mg/kg b.i.d., 50 mg/kg q.d., or 100 mg/kg q.d.] on joint inflammation and histopathology. Female Lewis rats (157-187 g) are anesthetized with isoflurane and injected with 300 µL Freund’s incomplete adjuvant, containing 2 mg/mL bovine type II collagen, at the base of the tail and two sites on the back on days 0 and 6. The rats are randomized to study groups at the onset of paw swelling (arthritis), which occurs between days 10 and 11. Dosing of either Decernotinib (VX-509; VRT-831509; adelatinib) or vehicle via oral gavage is initiated on the first day of established arthritis and continued to day 6 of arthritis. Dosing volume is 5 mL/kg. Groups are controls (no collagen injection plus vehicle; n = 4), collagen plus vehicle (n = 5), collagen plus Decernotinib (VX-509; VRT-831509; adelatinib) 10 mg/kg b.i.d. (n = 8); collagen plus Decernotinib (VX-509; VRT-831509; adelatinib) 10 mg/kg b.i.d. (n = 8); collagen plus (n = 8); collagen plus (n = 8); collagen plus (n = 8); collagen plu (n = 8); and collagen plus (n = 8); collagen plu (n = 8); all treatments are administered for 6 days. An additional group of rats is given collagen plus 10 mg/kg subcutaneous etanercept, a human tumor necrosis factor-α antagonist, on study days 11 and 14. Caliper measurements of normal (baseline) ankle joints begin on day 9 and continue through the last day of study. Differences in mean ankle diameter are tested for significance using Student’s t test, with significance set at P ≤ 0.05. The rats are euthanized on day 7 of arthritis, which is study day 17 or 18 depending on when animals are randomized to groups; paws and knees are harvested to determine paw weight and to conduct a histopathological analysis of inflammation (knee and ankle), pannus formation (ankle), cartilage destruction (knee), and bone resorption (knee and ankle). Scores range from 0 (normal) to 5 (severe pathology) and are assigned by a veterinary pathologist. Percent inhibition is calculated using the following formula: [(mean of treatment group) − (mean of control)] ÷ [(mean of collagen + vehicle) − (mean of control)]. Kruskal-Wallis one-way analysis of variance nonparametric tests are used to determine statistical significance among the histopathology groups, with significance set at P ≤ 0.05[1].
- In the mouse model of collagen - induced arthritis, mice were induced to develop arthritis, and then Decernotinib was administered orally at a dose of 1, 3, or 10 mg/kg, once a day for 21 days [1] - In the mouse model of oxazolone - induced colitis, mice were induced to develop colitis, and then Decernotinib was administered orally at a dose of 3 or 10 mg/kg, once a day for 7 days [1] CIA mouse protocol: 1. DBA/1J mice (male, 8–10 weeks old) were immunized subcutaneously with bovine type II collagen (100 μg in adjuvant) on day 0, and boosted on day 21. 2. When arthritis symptoms appeared (day 21, score ≥2), mice were randomized into 3 groups (n=6/group): - Vehicle: 0.5% methylcellulose in PBS, oral gavage, daily; - Decernotinib (VX-509) 10 mg/kg: dissolved in 0.5% methylcellulose, oral gavage, daily; - Decernotinib (VX-509) 30 mg/kg: same solvent and route as 10 mg/kg group. 3. Treatment lasted 28 days. Arthritis score (0–4 per limb, total 0–16) was measured daily. At euthanasia, hind joints were harvested for micro-CT (bone erosion analysis) and histopathology (cartilage loss); serum was collected for cytokine ELISA [1] - DTH mouse protocol: 1. BALB/c mice (female, 6–8 weeks old) were sensitized by subcutaneous injection of OVA (100 μg in adjuvant) on day 0. 2. On day 7, mice were challenged with OVA (50 μg in PBS) via intradermal injection in the right ear; the left ear received PBS. 3. Mice were treated with Decernotinib (VX-509) (5 mg/kg or 20 mg/kg, oral, daily) from day 0 to day 7. 4. On day 8, ear thickness was measured with a caliper (swelling = right ear thickness – left ear thickness). Ear tissue was homogenized to measure IFN-γ and IL-17 via ELISA [1] - EAE mouse protocol: 1. C57BL/6 mice (female, 6–8 weeks old) were immunized subcutaneously with MOG35-55 peptide (200 μg in adjuvant) on day 0, and received pertussis toxin (200 ng) intraperitoneally on day 0 and day 2. 2. On day 7 (onset of EAE symptoms), mice were randomized into 3 groups (n=6/group): - Vehicle: 0.5% methylcellulose, oral gavage, daily; - Decernotinib (VX-509) 15 mg/kg: oral gavage, daily; - Decernotinib (VX-509) 45 mg/kg: oral gavage, daily. 3. Treatment lasted 14 days. Clinical EAE score (0 = normal, 5 = moribund) was measured daily. At euthanasia, spinal cords were harvested for histology (inflammatory infiltrates and demyelination analysis) [1] |
| 药代性质 (ADME/PK) |
Oral bioavailability in rats: Male Sprague-Dawley rats (250–300 g) received Decernotinib (VX-509) via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg):
- Oral bioavailability = 62%;
- Oral administration: Cmax = 3.8 μg/mL (Tmax = 1.5 h), terminal half-life (t1/2) = 4.1 h, AUC0-24h = 20.5 μg·h/mL;
- Intravenous administration: Cmax = 9.2 μg/mL, t1/2 = 3.8 h, AUC0-∞ = 33.1 μg·h/mL [1]
- Plasma protein binding: In human plasma, Decernotinib (VX-509) had a protein binding rate of 92% (measured by equilibrium dialysis at 37°C) [1] - Tissue distribution in CIA mice: Oral Decernotinib (VX-509) (30 mg/kg) in CIA mice resulted in joint tissue concentration of 3.2 μg/g and spleen concentration of 4.5 μg/g at 2 h post-administration, ~1.1-fold and 1.5-fold of plasma concentration (2.9 μg/mL), respectively [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Rodent repeat-dose toxicity: Male/female Sprague-Dawley rats (n=4/sex/group) received Decernotinib (VX-509) (5 mg/kg, 30 mg/kg, 100 mg/kg, oral, daily) for 28 days:
- No mortality was observed; the no-observed-adverse-effect level (NOAEL) was 30 mg/kg;
- At 100 mg/kg: mild lymphopenia (lymphocyte count reduced by 22% vs. control), no histopathological changes in liver or kidneys, and unchanged serum ALT/AST/creatinine [1]
- In vivo safety in autoimmune models: In CIA, DTH, and EAE mice (highest dose 45 mg/kg, oral, up to 28 days): - No significant weight loss (<5%); - No overt toxicity (e.g., lethargy, diarrhea, reduced food intake); - Serum creatinine and BUN (renal function) remained within normal ranges [1] - In vitro normal cell safety: Human PBMCs and dermal fibroblasts treated with Decernotinib (VX-509) (≤500 nM) for 72 h showed >90% viability (MTT assay), with no significant apoptosis (Annexin V/PI staining) [1] |
| 参考文献 | |
| 其他信息 |
- Decernotinib is a JAK3 - specific inhibitor, which plays a role by inhibiting the JAK3 signaling pathway. It has potential therapeutic effects on autoimmune diseases, and the main mechanism is to reduce the production of pro - inflammatory cytokines by inhibiting JAK3, so as to alleviate the inflammatory response [1]
Decernotinib has been used in trials studying the treatment of Drug Interactions and Rheumatoid Arthritis. DECERNOTINIB is a small molecule drug with a maximum clinical trial phase of II and has 1 investigational indication. Cytokines, growth factors, and other chemical messengers rely on a class of intracellular nonreceptor tyrosine kinases known as Janus kinases (JAKs) to rapidly transduce intracellular signals. A number of these cytokines are critical for lymphocyte development and mediating immune responses. JAK3 is of particular interest due to its importance in immune function and its expression, which is largely confined to lymphocytes, thus limiting the potential impact of JAK3 inhibition on nonimmune physiology. The aim of this study was to evaluate the potency and selectivity of the investigational JAK3 inhibitor VX-509 (decernotinib) [(R)-2-((2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)-2-methyl-N-(2,2,2-trifluoroethyl)butanamide] against JAK3 kinase activity and inhibition of JAK3-mediated signaling in vitro and JAK3-dependent physiologic processes in vivo. These results demonstrate that VX-509 potently inhibits JAK3 in enzyme assays (Ki = 2.5 nM + 0.7 nM) and cellular assays dependent on JAK3 activity (IC50 range, 50-170 nM), with limited or no measurable potency against other JAK isotypes or non-JAK kinases. VX-509 also showed activity in two animal models of aberrant immune function. VX-509 treatment resulted in dose-dependent reduction in ankle swelling and paw weight and improved paw histopathology scores in the rat collagen-induced arthritis model. In a mouse model of oxazolone-induced delayed-type hypersensitivity, VX-509 reduced the T cell-mediated inflammatory response in skin. These findings demonstrate that VX-509 is a selective and potent inhibitor of JAK3 in vitro and modulates proinflammatory response in models of immune-mediated diseases, such as collagen-induced arthritis and delayed-type hypersensitivity. The data support evaluation of VX-509 for treatment of patients with autoimmune and inflammatory diseases such as rheumatoid arthritis.[1] Mechanism of action: Decernotinib (VX-509) exerts anti-inflammatory effects by selectively inhibiting JAK3, which is essential for signaling via the common γ-chain (γc) cytokines (e.g., IL-2, IL-4, IL-7, IL-15, IL-21). Inhibition of JAK3 blocks γc cytokine-mediated STAT5 phosphorylation, suppressing T-cell/B-cell activation, proliferation, and the production of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-17) [1] - Therapeutic potential: Preclinical data supports Decernotinib (VX-509) as a candidate for treating T-cell-mediated autoimmune diseases, including rheumatoid arthritis (RA), multiple sclerosis (MS), and psoriasis. Its high selectivity for JAK3 minimizes off-target effects associated with pan-JAK inhibitors (e.g., myelosuppression from JAK2 inhibition) [1] - Drug development context: Decernotinib (VX-509) was designed to address limitations of non-selective JAK inhibitors by targeting JAK3, a kinase specifically involved in adaptive immune responses, thereby reducing the risk of systemic side effects while maintaining anti-inflammatory efficacy [1] |
| 分子式 |
C18H19F3N6O
|
|
|---|---|---|
| 分子量 |
392.38
|
|
| 精确质量 |
392.157
|
|
| 元素分析 |
C, 55.10; H, 4.88; F, 14.53; N, 21.42; O, 4.08
|
|
| CAS号 |
944842-54-0
|
|
| 相关CAS号 |
|
|
| PubChem CID |
59422203
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
553.6±50.0 °C at 760 mmHg
|
|
| 闪点 |
288.6±30.1 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.603
|
|
| LogP |
2.26
|
|
| tPSA |
99.08
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
548
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
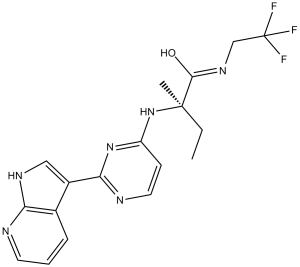
CC[C@](C)(C(=O)NCC(F)(F)F)NC1=NC(=NC=C1)C2=CNC3=C2C=CC=N3
|
|
| InChi Key |
ASUGUQWIHMTFJL-QGZVFWFLSA-N
|
|
| InChi Code |
InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1
|
|
| 化学名 |
(R)-2-((2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)-2-methyl-N-(2,2,2-trifluoroethyl)butanamide
|
|
| 别名 |
PubChem CID 59422203; VX-509; VRT831509 ; 944842-54-0; Adelatinib; Decernotinib (VX-509); Decernotinib(VX-509); VRT-831509; VX509; VX 509 ; VRT 831509 ; Decernotinib; Adelatinib
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5485 mL | 12.7427 mL | 25.4855 mL | |
| 5 mM | 0.5097 mL | 2.5485 mL | 5.0971 mL | |
| 10 mM | 0.2549 mL | 1.2743 mL | 2.5485 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01830985 | Completed | Drug: VX-509 | Rheumatoid Arthritis | Vertex Pharmaceuticals Incorporated | April 2013 | Phase 2 Phase 3 |
| NCT01886209 | Completed | Drug: Prednisone Drug: VX-509 |
Drug Interactions | Vertex Pharmaceuticals Incorporated | June 2013 | Phase 1 |
| NCT01754935 | Completed | Drug: VX-509 Drug: VX-509 matching placebo |
Rheumatoid Arthritis | Vertex Pharmaceuticals Incorporated | January 2013 | Phase 2 |
| NCT01590459 | Completed | Drug: VX-509 Drug: VX-509 matching placebo |
Rheumatoid Arthritis | Vertex Pharmaceuticals Incorporated | April 2012 | Phase 2 |
Effect of VX-509 on progression of established disease in rat CIA model.J Pharmacol Exp Ther.2015 May;353(2):405-14. |
(A) Representative histopathology photomicrographs of a control (collagen + vehicle) rat ankle and VX-509 b.i.d. treatment groups, with approximate mean score for group showing synovium (S), cartilage (large arrow), and bone (small arrow).J Pharmacol Exp Ther.2015 May;353(2):405-14. |
Effect of VX-509 on the oxazolone-induced mouse DTH model.J Pharmacol Exp Ther.2015 May;353(2):405-14. |