| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
| 靶点 |
Immunomodulator
|
|---|---|
| 体外研究 (In Vitro) |
化学处理诱导的细胞表面巯基的变化可能会影响膜蛋白的构象和细胞内信号机制。在我们之前的研究中,我们发现无毒剂量的Diphenylcyclopropenone/二苯基环丙烯酮(DPCP)是一种强效的皮肤增敏剂,可诱导人单核细胞系THP-1细胞中细胞表面硫醇的增加。在这里,我们研究了DPCP对细胞内信号传导的影响。首先,我们证实DPCP不仅在THP-1细胞中,而且在原代单核细胞中诱导细胞表面巯基的增加。细胞内还原型谷胱甘肽/氧化型谷胱甘肽比值(GSH/GSSG比值)不受DPCP处理的影响。通过用膜不可渗透的硫醇反应性化合物Alexa Fluor 488 C5马来酰亚胺(AFM)标记,然后进行二维凝胶电泳和液相色谱-电喷雾串联质谱(LC/MS/MS)分析,我们鉴定了几种硫醇含量因DPCP而改变的蛋白质。这些蛋白质包括细胞膜成分,如肌动蛋白和β-微管蛋白,分子伴侣,如热休克蛋白27A和70,以及内质网(ER)应激诱导蛋白。接下来,我们证实了剪接XBP1在DPCP处理的细胞中的表达,XBP1是ER应激的已知标志物。我们还检测了SAPK/JNK和p38 MAPK的磷酸化,它们是IRE1α-ASK1通路中的下游信号分子,由ER应激激活。这些数据表明,细胞表面巯基的增加可能与ER应激介导的信号传导的激活有关。[3]
Diphenylcyclopropenone/二苯基环丙烯酮(DPCP)局部免疫治疗被认为是治疗严重AA最有效的方法。然而,其机制尚不清楚,需要探索疗效的早期预测因素。TSLP/OX40L/IL-13通路是启动和维持Th2免疫反应的重要途径。我们之前的研究表明,这一途径可能在DPCP治疗的严重AA中发挥作用。因此,为了进一步研究经DPCP治疗的严重AA中TSLP/OX40L/IL-13通路的机制,并探索DPCP治疗疗效的预测因素,我们进行了一项前瞻性研究,比较了严重AA患者治疗前后TSLP、OX40L、Th2细胞因子IL-4、IL-5和IL13以及Th1细胞因子IFN-γ的表达水平。结果显示,21名AA患者对DPCP治疗有反应(反应者),12名患者没有反应(无反应者)。治疗前,应答者的TSLP、OX40L和IL-13水平低于无应答者。DPCP治疗后,应答者的TSLP、IL-5和IL-13增加,IFN-γ减少,而无应答者的TS LP、IL-4、IL-13和IFN-γ没有变化。我们的数据表明,TSLP/OX40L/IL-13通路在一些严重AA患者中下调,DPCP可能通过上调该通路在这些严重AA患者身上发挥治疗作用。TSLP/OX40L/IL-13通路可能是严重AA患者对DPCP治疗反应的预测因子[1]。 |
| 体内研究 (In Vivo) |
在用卵清蛋白(OVA)进行表皮免疫后,Diphenylcyclopropenone/二苯基环丙烯酮增强了抗原特异性IgG2a抗体反应以及IL-10细胞因子的产生。OVA和DCP的皮肤过敏原特异性免疫治疗(EPIT)也保护了致敏小鼠免受过敏反应和哮喘的侵害。这种保护作用比常规SCIT更强大,常规SCIT并没有显著缓解小鼠过敏反应和哮喘模型中的过敏症状。
对苯二胺(PPD)和Diphenylcyclopropenone/二苯基环丙烯酮(DPCP)是两种强效半抗原。已知这两种半抗原都会引起迟发型超敏反应,涉及细胞因子反应和T细胞亚群的局部浸润,导致接触性皮炎。我们研究了PPD和DPCP这两种相对未开发的皮肤过敏原的全身免疫作用。BALB/c小鼠耳朵的背侧暴露于PPD或DPCP(0.1%w/v或0.01%w/v)或单独的载体。小鼠每天治疗一次,持续3天(诱导期),随后每周治疗两次,持续8周。通过细胞因子谱MSD、流式细胞术和qPCR分析耳和胰腺淋巴结、脾脏、肝脏、血清和耳朵的局部和全身免疫反应。与赋形剂治疗相比,用1%PPD、0.01%DPCP或0.1%DPCP治疗的小鼠耳肿胀显著增加,表明小鼠已致敏,存在局部炎症。耳廓淋巴结、胰腺淋巴结、脾脏和肝脏显示调节性T细胞、B细胞和NKT细胞频率的变化,CD8+T细胞和B细胞的活化增加。细胞内细胞因子分析显示,用两种半抗原治疗后,肝脏中存在的IFN-γ和IL-4阳性NKT细胞增加。此外,我们观察到IL-17A有全身性增加的趋势。我们观察了PPD和DPCP的全身免疫效应。此外,浓度太低而不能增加耳朵厚度并引起临床症状,仍可能激活免疫系统。这些全身免疫效应可能使个体易患某些疾病[4]。 背景/目的:斑秃(AA)是一种具有遗传和自身免疫基础的炎症性疾病。本文旨在研究免疫调节治疗剂Diphenylcyclopropenone/二苯基环丙烯酮的疗效和安全性,同时揭示其与组织病理学特征、预后因素和副作用的关联。 材料和方法:在这项回顾性研究中,纳入了2011年至2015年间转诊到皮肤科毛发疾病综合诊所的98名脱发患者(60名男性,38名女性)。结合病史和皮肤病学检查,在治疗前对所有患者进行了皮肤活检以进行组织病理学检查。根据毛发再生百分比评估治疗成功率。 结果:关于总体治疗成功率,33名(34%)患者完全缓解,16名(16%)患者部分缓解(50%至99%),27名(28%)患者反应轻微(1%至49%),22名(22%)患者无反应。结果中,男女比例相等。 结论:脱发的严重程度与治疗结果之间存在显著相关性(P=0.038)。与全秃和全秃患者相比,AA患者的反应明显更好。与其他参数,如疾病持续时间、年龄、性别、特应性病史、发病年龄和组织病理学特征,没有统计学上的显著相关性[2]。 |
| 细胞实验 |
化学处理诱导的细胞表面巯基的变化可能会影响膜蛋白的构象和细胞内信号机制。在我们之前的研究中,我们发现无毒剂量的二苯基环丙烯(DPCP)是一种强效的皮肤增敏剂,可诱导人单核细胞系THP-1细胞中细胞表面硫醇的增加。在这里,我们研究了DPCP对细胞内信号传导的影响。首先,我们证实DPCP不仅在THP-1细胞中,而且在原代单核细胞中诱导细胞表面巯基的增加。细胞内还原型谷胱甘肽/氧化型谷胱甘肽比值(GSH/GSSG比值)不受DPCP处理的影响。通过用膜不可渗透的硫醇反应性化合物Alexa Fluor 488 C5马来酰亚胺(AFM)标记,然后进行二维凝胶电泳和液相色谱-电喷雾串联质谱(LC/MS/MS)分析,我们鉴定了几种硫醇含量因DPCP而改变的蛋白质。这些蛋白质包括细胞膜成分,如肌动蛋白和β-微管蛋白,分子伴侣,如热休克蛋白27A和70,以及内质网(ER)应激诱导蛋白。接下来,我们证实了剪接XBP1在DPCP处理的细胞中的表达,XBP1是ER应激的已知标志物。我们还检测了SAPK/JNK和p38 MAPK的磷酸化,它们是IRE1α-ASK1通路中的下游信号分子,由ER应激激活。这些数据表明,细胞表面巯基的增加可能与ER应激介导的信号传导的激活有关[3]。
|
| 动物实验 |
In a mouse model of allergy, we tested the adjuvant potential of diphenylcyclopropenone (DCP), a strong contact sensitizer, which is currently used for the treatment of a T cell-mediated hair loss disease (alopezia areata).[3]
Female CBA mice were used at the age of 6–8 weeks. Before epicutaneous immunization, mice were shaved on their belly (2 × 2 cm). After 4 h, the mice were anaesthetized with xylazin 8 mg/kg and ketamine 50 mg/kg intraperitoneally (i.p.) 13. The skin was tape stripped 10 times with a scotch tape. Thereafter, the mice were immunized with 25-μg ovalbumin (OVA; Grade V) dissolved in water/acetone/dibutylphthalate (1 : 1 : 2) ± 1% DCP. The vaccination was repeated after 7, 14, and 28 days (Fig. 1A). Sham controls were treated with the vehicle with 1% DCP. For reference, some mice were immunized four times subcutaneously with 25-μg OVA in PBS/aluminum hydroxide (2 : 1). Hapten treatment for mice and measurement of ear thickness [4] The mice were sedated with hypnome/midazolam (0.01 μl per gram), and weight and ear thickness were measured prior to the first application of hapten. The haptens were dissolved in the vehicle acetone/olive oil 4:1 (AOO), and the mice were exposed on the dorsal sides of both ears to 25 μl of the respective hapten, p-Phenylenediamine, Diphenylcyclopropenone, or vehicle. For the PPD groups, the concentrations were 1.0 or 0.1 % (w/v). For the Diphenylcyclopropenone/DPCP groups, the concentrations were 0.1 or 0.01 %. Sensitization was achieved by treatment once daily for 3 days followed by challenge twice per week for 8 weeks. The mice were euthanized by cervical dislocation 24 h after the last treatment, and the draining auricular lymph nodes (ALN), pancreatic lymph nodes (PLN), spleen, liver, ears, and pancreas were removed and used for flow cytometry and quantitative PCR. Serum samples were collected by jaw puncture prior to euthanization. Treatment method with Diphenylcyclopropenone/DPCP [2] Topical immunotherapy with Diphenylcyclopropenone/DPCP was performed following a standard protocol of sensitization. This protocol comprised the application of a 2% concentration of DPCP solution diluted in acetone, over an area of 2 × 2 cm on the occipital region of the scalp. The patients were told to avoid water contact of the sensitized area for 2 days and avoid sun exposure by using a wig or protective hat. After 2 days, the patients were checked to detect whether or not sensitization to DPCP had occurred. There was a lag period of 2 weeks after the sensitization, and then the treatment began. The initial DPCP concentration was 0.001%. DPCP solution was applied to all of the affected areas on the scalp, together with the eyebrows, once every week. Again after each session, the patients were told to avoid water contact for 2 days, including excessive sweating and sun exposure. DPCP solution was left on the scalp for 48 h and then washed off with a mild shampoo. The concentration of the DPCP solutions were increased weekly, unless there were serious side effects, including irritant contact dermatitis and photoallergic reactions, and the final concentration of 2% was reached at the end of week 6. Concentrations of 0.001%, 0.01%, 0.1%, 0.2%, 0.5%, 1%, and 2% were applied sequentially. Follow-up and assessment of efficacy [2] During follow-up visits, the side effects of the patients were recorded, as well as the grade of hair regrowth. Once complete or cosmetically acceptable hair regrowth (amount of growth that eliminated the need for using a wig or hat) was achieved, the intervals of the Diphenylcyclopropenone/DPCP application were prolonged to 2 weeks, 3 weeks, and monthly. By this method, the DPCP immunotherapy was discontinued gradually. In the case of hair loss during this tapering-off period, therapy was restored at weekly intervals. If there was no obvious response at the end of 6 months, immunotherapy was considered as noneffective and discontinued. |
| 毒性/毒理 (Toxicokinetics/TK) |
Many adverse events have been reported due to the use of Diphenylcyclopropenone/DPCP, including eczematous reactions, urticaria, vitiligo, lymphadenopathy, hyperpigmentation, or erythema multiforme-like reactions. In the current study, during the sensitization process, almost all of the patients experienced minimal erythema, itching, and burning sensation. Overall, the most commonly encountered side effect was erythema and itching, followed by the formation of papules, vesicles, bullae, and flu-like symptoms. Other less frequent side effects were lymph node enlargement, fever, general malaise, irreversible hyperpigmentation of the head and neck area, and vitiligo macules.
The use of DPCP in children is still a controversial area, although some studies have shown good results with acceptable side effect profiles. In the current study, children above 5 years of age were also included and the treatment results or safety parameters were similar to those of the adult patients.[2] A total of 33 severe AA patients and 20 healthy volunteers (normal controls) were enrolled. There were no statistical differences between AA patients and normal controls for age and gender. All the patients were treated with Diphenylcyclopropenone/DPCP, 21 (63.6%) had satisfactory hair regrowth (responders), and 12 (36.4%) were not responsive to the treatment (non-responders). Information of patients is presented (Table 1). No significant differences in gender, age, onset age, disease duration and SALT scores were found between responders and non-responders (Table 1). No significant associations were found between responses to the treatment and the final concentration of DPCP/Diphenylcyclopropenone applied, treatment reaction, side effects, nail involvement and other clinical features (data not shown). A total of 30 patients (90.9%) experienced one or more side effects during the treatment. Dermatitis in the Diphenylcyclopropenone/DPCP contact area was the most common finding (27/30, 90%). Lymphadenopathy in the draining area was another common side effect (14/30, 46.7%). Other side effects included scalp hyperpigmentation (12/30, 40.0%), systemic contact dermatitis (5/30, 16.7%), scalp hypopigmentation (2/30, 6.7%) and hyperpyrexia (1/30, 3.3%). Side effects resolved after application of topical corticosteroids, use of systemic antihistamines or treatment suspension for one week, but they did not lead to discontinuation of therapy.[1] |
| 参考文献 |
[1]. Diphenylcyclopropenone plays an effective therapeutic role by up-regulating the TSLP/OX40L/IL-13 pathway in severe alopecia areata. Exp Dermatol. 2021 Feb;30(2):278-283.
[2]. ssessment of treatment efficacy of diphenylcyclopropenone (DPCP) for alopecia areata. Turk J Med Sci. 2020 Dec 17;50(8):1817-1824. [3]. Changes of cell-surface thiols and intracellular signaling in human monocytic cell line THP-1 treated with diphenylcyclopropenone. J Toxicol Sci. 2010 Dec;35(6):871-9. [4]. The contact sensitizer diphenylcyclopropenone has adjuvant properties in mice and potential application in epicutaneous immunotherapy. Allergy. 2012 May;67(5):638-46. |
| 其他信息 |
Diphenylcyclopropenone is a cyclopropenone compound having phenyl substituents at the 2- and 3-positions. It has a role as a photosensitizing agent, a hapten and a drug allergen.
Diphencyprone has been used in trials studying the treatment and basic science of Melanoma, Ultraviolet Rays, Immunosuppression, Neoplasm Metastasis, and Hypersensitivity, Delayed, among others. Diphencyprone is a synthetic, potent allergic contact sensitizer with potential immunostimulatory activity. After sensitization process by repeated topical application of diphencyprone to a specific area, further application of this agent to the affected area may stimulate an immune response and may potentially be useful to clear the affected area from infection or cancer. To our knowledge, this is the first study investigating the expression of the TSLP/OX40L/IL13 pathway in severe AA patients and immunotherapy with DPCP. Results showed that expression levels of Th2 cytokines IL-5 and TSLP were significantly lower and Th1 cytokine IFN-γ was higher in serum of severe AA patients than normal. It was similar in the scalp skin. TSLP, OX40L, IL-4 and IL-13 were found to be much lower, and IFN-γ was higher than the normal control, which is consistent with the previous report detecting mRNA levels of Th1-type cytokines in scalp skin. Results of both serum and scalp skin suggest that the TSLP/OX40L/IL13 pathway is down-regulated, Th2 responses are inhibited and Th1 immune is dominant in severe AA patients. Down-regulation of the TSLP/OX40L/IL13 pathway reflects a lower Th2 activity in severe AA patients. Therefore, as an effective treatment for severe AA, DPCP might play its role in the process by up-regulating the TSLP/OX40L/IL13 pathway, promoting Th2 immune activity and then restoring equilibrium between Th1 and Th2 responses. We compared expression levels of related cytokines before and after the DPCP treatment. It was shown that TSLP, IL-13 and another Th2 cytokine IL-5 significantly increased whereas Th1 cytokine IFN-γ decreased to a normal level in severe AA patients after being treated with DPCP. The result proves that DPCP plays a therapeutic role in severe AA by up-regulating the TSLP/OX40L/IL13 pathway and reconstructing Th1/Th2 immune balance. Topical immunotherapy with DPCP is considered to be an effective and safe therapeutic method for AA, but not all patients respond to it. In this study, 12 out of 33 (36.36%) AA patients did not respond to the therapy. Was there any difference between responders and non-responders which leads to the different responses to the DPCP therapy? Comparing the expression levels of Th2- and Th1-related cytokines between the responder and non-responder groups, we found that before the DPCP treatment, the expression levels of TSLP, OX40L and IL-13 in responders were significantly lower than non-responders, while non-responders had similar expression levels of these cytokines with normal controls. After being treated with DPCP, TSLP and IL-13 in serum greatly increased in responders while there were no changes of these cytokines in non-responders. There were no significant differences of Th1 cytokine IFN-γ and other Th2 cytokines IL-5 and IL-4 between the responder and non-responder groups, no matter before or after the DPCP treatment. These results indicate that the difference between the responders and the non-responders is in the TSLP/OX40L/IL-13 pathway. Patients with down-regulation of the TSLP/OX40L/IL-13 pathway were responsive to the DPCP treatment while those who without down-regulation of the pathway were not responsive. The results further demonstrate that DPCP plays its therapeutic role by up-regulating TSLP/OX40L/IL-13 pathway in some severe AA patients. In conclusion, this study shows that the TSLP/OX40L/IL-13 pathway is down-regulated and Th2 immune is inhibited in some severe AA patients. Topical treatment with DPCP is only effective in patients with down-regulation of the TSLP/OX40L/IL-13 pathway. The TSLP/OX40L/IL-13 pathway could be a predictor of response to DPCP therapy for severe AA patients.[1] |
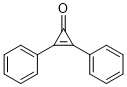
| 分子式 |
C15H10O
|
|---|---|
| 分子量 |
206.244
|
| 精确质量 |
206.073
|
| 元素分析 |
C, 87.36; H, 4.89; O, 7.76
|
| CAS号 |
886-38-4
|
| 相关CAS号 |
886-38-4;
|
| PubChem CID |
65057
|
| 外观&性状 |
Typically exists as Off-white to light yellow solids at room temperature
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
407.2±45.0 °C at 760 mmHg
|
| 熔点 |
118-122 °C(lit.)
|
| 闪点 |
182.7±23.7 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.669
|
| LogP |
3.78
|
| tPSA |
17.07
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
284
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C(C2C=CC=CC=2)=C1C1C=CC=CC=1
|
| InChi Key |
HCIBTBXNLVOFER-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H10O/c16-15-13(11-7-3-1-4-8-11)14(15)12-9-5-2-6-10-12/h1-10H SMILES
|
| 化学名 |
2,3-Diphenylcycloprop-2-en-1-one
|
| 别名 |
Diphencyprone; DPCP; Diphenylcyclopropenone; Diphencyprone; 2,3-Diphenylcycloprop-2-en-1-one; 2,3-Diphenylcycloprop-2-enone; 2,3-Diphenylcyclopropenone; 1,2-Diphenylcyclopropen-3-one; Cyclopropenone, diphenyl-;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~484.87 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (12.12 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8487 mL | 24.2436 mL | 48.4872 mL | |
| 5 mM | 0.9697 mL | 4.8487 mL | 9.6974 mL | |
| 10 mM | 0.4849 mL | 2.4244 mL | 4.8487 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05481658 | RECRUITING | Drug: Diphencyprone (DPCP) | Cutaneous Metastases | Nicholas Gulati | 2022-10-06 | Phase 1 |
| NCT04775979 | COMPLETED | Drug: diphenylcyclopropenone (DPCP) | Vitiligo | Ain Shams University | 2021-01-17 | Phase 4 |
| NCT01452594 | COMPLETED | Drug: Diphenylcyclopropenone Drug: Placebo |
Healthy Volunteers | Rockefeller University | 2011-10 | |
| NCT05438290 | COMPLETED | Drug: DPCP | Cutaneous Neurofibroma | Nicholas Gulati | 2022-09-14 | Phase 1 |
| NCT01711684 | COMPLETED | Drug: Diphenylcyclopropenone (DPCP) | Melanoma Neoplasm Metastasis |
Rockefeller University | 2012-10-16 | Phase 1 |