| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Diroximel fumarate is rapidly absorbed in the gastrointestinal tract following administration, like its bioequivalent drug, dimethyl fumarate. The median Tmax of monomethyl fumarate (MMF) after oral administration ranges from 2.5-3 hours with a mean Cmax of 2.11 mg/L. The bioequivalent drug, dimethyl fumarate, administered to healthy volunteers also shows a similar mean Tmax and Cmax. The average steady state concentration of this metabolite is estimated at 8.32 mg.hr/L after it is administered twice a day in patients with MS. The mean AUC0–∞ of the active metabolite is 88mg × min L−1. Food appears to significantly reduce the Cmax of diroximel fumarate's active metabolite, MMF, when compared to administration in the fasted state. Monomethyl fumarate is eliminated as carbon dioxide through expired breath. Negligible amounts, under 0.3% of the ingested dose, are measured in urine. The inactive metabolite, 2-hydroxyethyl succinimide (HES), representing 58-63% of the ingested dose, is excreted in urine. The apparent volume of distribution ranges from 72L to 83L. Monomethyl fumarate (MMF), the active metabolite of diroximel fumarate, crosses the blood brain barrier. No clearance information is available on the FDA label for diroximel fumarate, however, clinical study results for its active metabolite, monomethyl fumarate show a mean apparent total clearance from the plasma after oral administration of 1.54 mgL−1. Metabolism / Metabolites Esterases heavily metabolize diroximel fumarate, as well as its bioequivalent drug, dimethyl fumarate, in the liver. These enzymes are present in high quantities in the gastrointestinal tract, tissues, and blood. Esterase metabolism of this drug produces the active metabolite, mono methyl fumarate (MMF), before it moves to the systemic circulation. In addition, the major inactive metabolite, 2-hydroxyethyl succinimide (HES) is produced along with small amounts of methanol, and another inactive metabolite, RDC-8439. Following esterase metabolism, the tricarboxylic acid (TCA)cycle further metabolizes MMF. The major metabolites of MMF in plasma include fumaric acid, citric acid, and glucose. It is important that methanol is a major metabolite of dimethyl fumarate metabolism, but a minor metabolite of diroximel fumarate metabolism, conferring its lower risk of gastrointestinal effects. Biological Half-Life The terminal half-life of monomethyl fumarate (MMF), diroximel fumarate's active metabolite, is estimated to be 1 hour. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of diroximel fumarate during breastfeeding. However, amounts of the active metabolite of diroximel fumarate, monomethyl fumarate, in breastmilk appear to be low and would not be expected to cause any adverse effects in breastfed infants. Based on clinical data in over 20 infants exposed to dimethyl fumarate in breastmilk, diroximel fumarate is acceptable to use during breastfeeding, at least after one month of age. Breastfed infants should be monitored for adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants. Some authors also recommend monitoring breastfed infants for flushing, vomiting and diarrhea. ◉ Effects in Breastfed Infants Twenty-six women taking dimethyl fumarate for relapsing-remitting multiple sclerosis were followed during 29 pregnancies from 2015 to 2020. Dimethyl fumarate was administered through week 24 of pregnancy and resumed 1 month after delivery. Twenty-two of 26 mothers breastfed (extent not stated) for 4 to 7 months. Infants were monitored up to the third year of life for infections and developmental disorders. All children were the 70th and 95th percentile for height and weight. The authors concluded that continuing dimethyl fumarate while breastfeeding is safe, although infants were not exposed during the first month of life. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding of MMF, the active metabolite of diroximel fumarate, ranges from 27-45%. |
| 参考文献 | |
| 其他信息 |
Multiple Sclerosis (MS) is a chronic, debilitating neurological disease that can lead to profound cognitive and physical symptoms, severely affecting quality of life. It is the main cause of neurological disability not caused by trauma in the young adult population of both North America and Europe. Relapsing-remitting forms of MS lead to neurological symptoms that resolve and recur periodically. More than 80% of patients suffering from this disease have relapsing-remitting MS. Diroximel fumarate is a new drug from the fumarate class formulated to treat various relapsing forms of MS. This drug is bioequivalent to [Dimethyl fumarate](initially manufactured in 2013), but is less likely to cause gastrointestinal side effects, owing to its unique chemical structure. Diroximel fumarate was formulated by Alkermes in collaboration with Biogen, and was approved by the FDA in October 2019 and by the EMA in November 2021.
See also: Monomethyl Fumarate (has active moiety). Drug Indication Diroximel fumarate is indicated for the treatment of relapsing forms of multiple sclerosis (MS) in adults; specifically active secondary progressive disease and clinically isolated syndrome, as well as relapsing-remitting MS. Vumerity is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis (see Section 5. 1 for important information on the populations for which efficacy has been established). Mechanism of Action Currently, the mechanism of action of this drug in MS is not fully understood. Diroximel fumarate is hypothesized to regulate cell signaling pathways, causing beneficial immune and neuroprotective effects. Monomethyl fumarate (MMF) is the active metabolite of diroximel fumarate, and activates the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in humans. This pathway occurs as a response to oxidative stress in cells. In addition to the above, MMF is a nicotinic acid receptor agonist in the laboratory setting. The relevance of this finding to the treatment of MS is unknown at this time. The mechanism by which this drug leads to less gastrointestinal effects is purported to be due to its lack of a methanol leaving group in its chemical structure, and substitution with inert 2-hydroxyethyl succinimide. Pharmacodynamics Diroximel fumarate relieves the neurological symptoms of relapsing MS with less gastrointestinal effects than its bioequivalent counterpart, dimethyl fumarate. It is important to note that diroximel fumarate can cause angioedema, anaphylaxis, hepatotoxicity, flushing, lymphopenia, and Progressive Multifocal Leukoencephalopathy (PML). Discontinue diroximel fumarate immediately if PML is suspected or if anaphylaxis or angioedema occur. Liver function and total bilirubin should be tested prior to initiating diroximel fumarate and during treatment. A complete blood count (CBC) should be obtained prior to starting diroximel fumarate, after the first 6 months of administration, and at subsequent intervals of 6 to 12 months following this period. Suspend treatment if lymphocyte counts are measured to be less than 0.5 × 109/L for more than 6 months. |
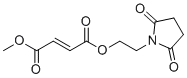
| 分子式 |
C11H13NO6
|
|---|---|
| 分子量 |
255.224023580551
|
| 精确质量 |
255.074
|
| CAS号 |
1577222-14-0
|
| PubChem CID |
73330464
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
441.0±25.0 °C at 760 mmHg
|
| 熔点 |
102-106
|
| 闪点 |
220.5±23.2 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.515
|
| LogP |
-0.24
|
| tPSA |
90
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
384
|
| 定义原子立体中心数目 |
0
|
| SMILES |
COC(=O)/C=C/C(=O)OCCN1C(=O)CCC1=O
|
| InChi Key |
YIMYDTCOUQIDMT-SNAWJCMRSA-N
|
| InChi Code |
InChI=1S/C11H13NO6/c1-17-10(15)4-5-11(16)18-7-6-12-8(13)2-3-9(12)14/h4-5H,2-3,6-7H2,1H3/b5-4+
|
| 化学名 |
2-(2,5-dioxopyrrolidin-1-yl)ethyl methyl fumarate
|
| 别名 |
ALKS-8700; BIIB-098; VUMERITY™; ALKS8700; ALKS 8700; ALKS-8700
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~489.77 mM)
H2O : ≥ 10 mg/mL (~39.18 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (8.15 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (8.15 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (8.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9182 mL | 19.5909 mL | 39.1819 mL | |
| 5 mM | 0.7836 mL | 3.9182 mL | 7.8364 mL | |
| 10 mM | 0.3918 mL | 1.9591 mL | 3.9182 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。