| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
dynamin (IC50 = 15 μM); HSV-1/2
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
Dynasore 抑制 dynamin1、dynamin2 和线粒体 dynamin Drp1 GTPase 活性,但不抑制其他小 GTPase。添加后,dynasore 会迅速阻止包被囊泡的形成,从而充当已知的动力依赖性内吞途径的强大抑制剂。这种效果在几秒钟内就可以看到。 G 形半成形凹坑和 O 形完全成形凹坑(在夹断过程中截留)是 dynasore 加工过程中积累的两种类型的涂层凹坑中间体 [1]。人类胎儿神经元、星形胶质细胞、初级生殖道细胞以及上皮细胞和神经细胞都容易受到HSV-1和HSV-2感染。 Dynasore 可以预防这些感染。病毒进入后八小时给予 dynasore 可以防止新生成的病毒蛋白离开细胞核,并增加到达核孔的病毒衣壳数量 [2]。 Dynasore 抑制缺血或再灌注引起的左心室舒张末压升高。此外,Dynamicore 可减少再灌注时的梗塞面积和心肌肌钙蛋白 I 流出。 Dynasore 增加了氧化应激下生长的成年小鼠心肌细胞的活力和存活率 [3]。
|
||
| 体内研究 (In Vivo) |
大鼠脊髓损伤 (SCI) 后,dynasore 在第 3、7 和 10 天显着减少了运动功能障碍。通过防止脊髓损伤后大鼠神经元中导致线粒体凋亡和星形胶质细胞增殖的途径激活,Dynasore 极大地改善了运动功能 [4]。
|
||
| 酶活实验 |
ATP测量[3]
发光分析仪用于定量心肌细胞和Hela细胞ATP含量。简而言之,在Dynasore治疗和H2O2暴露后,心肌细胞被裂解,细胞裂解物中的ATP含量被测量。同时,在遵循相同实验方案的另一组孔中,使用TBE测定法对存活的心肌细胞进行计数。然后计算每种治疗条件下每个活心肌细胞的细胞ATP。类似的程序也应用于用对照或Dynasore处理的培养的非应激Hela细胞。 |
||
| 细胞实验 |
成年小鼠心肌细胞的分离与培养[3]
使用先前描述的方法用胶原酶II(2mg/ml)分离后,从雄性成年C6/黑小鼠(8-12周)中分离出小鼠心室肌细胞。分离后,将心肌细胞以约1500/mm2的密度铺在层粘连蛋白预涂的35mm2培养皿上,并在37°C下保持在5%CO2的加湿气氛中。接种1小时后,用新鲜培养基(补充或耗尽血清)补充心肌细胞,并进行2小时的药物治疗(Dynasore或赋形剂),然后进行氧化应激(30µM H2O2 35分钟)。对于ATP补充实验,细胞在暴露于H2O2之前用3 mM ATP处理30分钟。 旋转圆盘共聚焦显微镜活细胞线粒体成像[3] HeLa细胞在添加了10%FBS和100µg/ml Normocin的DMEM中维持。细胞在37°C、5%CO2的加湿气氛中保持。以7×104个细胞/cm2的密度接种细胞,并使其粘附过夜。然后用Organille Lights™Mito RFP*BacMam 1.0转导细胞。转导后24小时,细胞用对照或1µM Dynasore预处理1小时,然后暴露于正常条件或200µM H2O2 15分钟。在暴露于H2O2之前和之后,使用尼康Ti倒置显微镜、横河CSU-X1旋转盘共聚焦单元和568nm DPSS激光源以及高分辨率Cool SNAP HQ2相机对细胞进行成像。图像以每帧400毫秒的曝光量采集,并使用浅浮雕滤镜自动处理以突出边缘。 |
||
| 动物实验 |
|
||
| 参考文献 | |||
| 其他信息 |
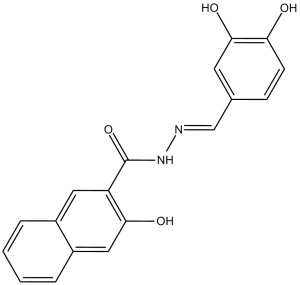
Dynasore is a carbohydrazide resulting from the formal condensation of the hydrazone moiety of 3,4-dihydroxybenzaldehyde hydrazone with the carboxy group of 3-hydroxy-2-naphthoic acid. It is a cell-permeable, reversible noncompetitive inhibitor of the GTPase activity of dynamin 1 and 2 and Drp1 (mitochondrial), while exhibiting no significant effect against two other small GTPases, MxA and Cdc42. It has a role as an EC 3.6.5.5 (dynamin GTPase) inhibitor. It is a member of catechols, a member of naphthols, a hydrazide and a hydrazone. It is functionally related to a 3-Hydroxy-2-naphthoate.
Dynamin is essential for clathrin-dependent coated vesicle formation. It is required for membrane budding at a late stage during the transition from a fully formed pit to a pinched-off vesicle. Dynamin may also fulfill other roles during earlier stages of vesicle formation. We have screened about 16,000 small molecules and have identified 1, named here dynasore, that interferes in vitro with the GTPase activity of dynamin1, dynamin2, and Drp1, the mitochondrial dynamin, but not of other small GTPases. Dynasore acts as a potent inhibitor of endocytic pathways known to depend on dynamin by rapidly blocking coated vesicle formation within seconds of dynasore addition. Two types of coated pit intermediates accumulate during dynasore treatment, U-shaped, half formed pits and O-shaped, fully formed pits, captured while pinching off. Thus, dynamin acts at two steps during clathrin coat formation; GTP hydrolysis is probably needed at both steps.[1] Dynasore, a small-molecule inhibitor of the GTPase activity of dynamin, inhibits the entry of several viruses, including herpes simplex virus (HSV), but its impact on other steps in the viral life cycle has not been delineated. The current study was designed to test the hypothesis that dynamin is required for viral protein trafficking and thus has pleiotropic inhibitory effects on HSV infection. Dynasore inhibited HSV-1 and HSV-2 infection of human epithelial and neuronal cells, including primary genital tract cells and human fetal neurons and astrocytes. Similar results were obtained when cells were transfected with a plasmid expressing dominant negative dynamin. Kinetic studies demonstrated that dynasore reduced the number of viral capsids reaching the nuclear pore if added at the time of viral entry and that, when added as late as 8 h postentry, dynasore blocked the transport of newly synthesized viral proteins from the nucleus to the cytosol. Proximity ligation assays demonstrated that treatment with dynasore prevented the colocalization of VP5 and dynamin. This resulted in a reduction in the number of viral capsids isolated from sucrose gradients. Fewer capsids were observed by electron microscopy in dynasore-treated cells than in control-treated cells. There were also reductions in infectious progeny released into culture supernatants and in cell-to-cell spread. Together, these findings suggest that targeting dynamin-HSV interactions may provide a new strategy for HSV treatment and prevention. Importance: HSV infections remain a global health problem associated with significant morbidity, particularly in neonates and immunocompromised hosts, highlighting the need for novel approaches to treatment and prevention. The current studies indicate that dynamin plays a role in multiple steps in the viral life cycle and provides a new target for antiviral therapy. Dynasore, a small-molecule inhibitor of dynamin, has pleiotropic effects on HSV-1 and HSV-2 infection and impedes viral entry, trafficking of viral proteins, and capsid formation.[2] Background: Heart failure due to diastolic dysfunction exacts a major economic, morbidity and mortality burden in the United States. Therapeutic agents to improve diastolic dysfunction are limited. It was recently found that Dynamin related protein 1 (Drp1) mediates mitochondrial fission during ischemia/reperfusion (I/R) injury, whereas inhibition of Drp1 decreases myocardial infarct size. We hypothesized that Dynasore, a small noncompetitive dynamin GTPase inhibitor, could have beneficial effects on cardiac physiology during I/R injury. Methods and results: In Langendorff perfused mouse hearts subjected to I/R (30 minutes of global ischemia followed by 1 hour of reperfusion), pretreatment with 1 µM Dynasore prevented I/R induced elevation of left ventricular end diastolic pressure (LVEDP), indicating a significant and specific lusitropic effect. Dynasore also decreased cardiac troponin I efflux during reperfusion and reduced infarct size. In cultured adult mouse cardiomyocytes subjected to oxidative stress, Dynasore increased cardiomyocyte survival and viability identified by trypan blue exclusion assay and reduced cellular Adenosine triphosphate(ATP) depletion. Moreover, in cultured cells, Dynasore pretreatment protected mitochondrial fragmentation induced by oxidative stress. Conclusion: Dynasore protects cardiac lusitropy and limits cell damage through a mechanism that maintains mitochondrial morphology and intracellular ATP in stressed cells. Mitochondrial protection through an agent such as Dynasore can have clinical benefit by positively influencing the energetics of diastolic dysfunction.[3] Spinal cord injury (SCI) is a common and devastating central nervous system insult which lacks efficient treatment. Our previous experimental findings indicated that dynamin-related protein 1 (Drp1) mediates mitochondrial fission during SCI, and inhibition of Drp1 plays a significant protective effect after SCI in rats. Dynasore inhibits GTPase activity at both the plasma membrane (dynamin 1, 2) and the mitochondria membrane (Drp1). The aim of the present study was to investigate the beneficial effects of dynasore on SCI and its underlying mechanism in a rat model. Sprague-Dawley rats were randomly assigned to sham, SCI, and 1, 10, and 30 mg dynasore groups. The rat model of SCI was established using an established Allen's model. Dynasore was administered via intraperitoneal injection immediately. Results of motor functional test indicated that dynasore ameliorated the motor dysfunction greatly at 3, 7, and 10 days after SCI in rats (P < 0.05). Results of western blot showed that dynasore has remarkably reduced the expressions of Drp1, dynamin 1, and dynamin 2 and, moreover, decreased the Bax, cytochrome C, and active Caspase-3 expressions, but increased the expressions of Bcl-2 at 3 days after SCI (P < 0.05). Notably, the upregulation of proliferating cell nuclear antigen (PCNA) and glial fibrillary acidic protein (GAFP) are inhibited by dynasore at 3 days after SCI (P < 0.05). Results of immunofluorescent double labeling showed that there were less apoptotic neurons and proliferative astrocytes in the dynasore groups compared with SCI group (P < 0.05). Finally, histological assessment via Nissl staining demonstrated that the dynasore groups exhibited a significantly greater number of surviving neurons compared with the SCI group (P < 0.05). This neuroprotective effect was dose-dependent (P < 0.05). To our knowledge, this is the first study to indicate that dynasore significantly enhances motor function which may be by inhibiting the activation of neuronal mitochondrial apoptotic pathway and astrocytic proliferation in rats after SCI.[4] |
| 分子式 |
C18H14N2O4
|
|
|---|---|---|
| 分子量 |
322.31
|
|
| 精确质量 |
322.095
|
|
| 元素分析 |
C, 67.08; H, 4.38; N, 8.69; O, 19.85
|
|
| CAS号 |
304448-55-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
135533054
|
|
| 外观&性状 |
Light brown to brown solid powder
|
|
| 密度 |
1.36±0.1 g/cm3
|
|
| 折射率 |
1.665
|
|
| LogP |
4.06
|
|
| tPSA |
102.15
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
24
|
|
| 分子复杂度/Complexity |
470
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
C1=CC=C2C=C(C(=CC2=C1)C(=O)N/N=C/C3=CC(=C(C=C3)O)O)O
|
|
| InChi Key |
SYNDQCRDGGCQRZ-VXLYETTFSA-N
|
|
| InChi Code |
InChI=1S/C18H14N2O4/c21-15-6-5-11(7-17(15)23)10-19-20-18(24)14-8-12-3-1-2-4-13(12)9-16(14)22/h1-10,21-23H,(H,20,24)/b19-10+
|
|
| 化学名 |
(E)-N-(3,4-dihydroxybenzylidene)-3-hydroxy-2-naphthohydrazide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.76 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.76 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1026 mL | 15.5130 mL | 31.0260 mL | |
| 5 mM | 0.6205 mL | 3.1026 mL | 6.2052 mL | |
| 10 mM | 0.3103 mL | 1.5513 mL | 3.1026 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|