| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
RIPK1 (receptor-interacting protein kinase 1) (IC50 <1 µM)
|
|---|---|
| 体外研究 (In Vitro) |
Eclitasertib(DNL758;SAR443122)是一种受体相互作用的丝氨酸/苏氨酸蛋白(RIP-1)激酶抑制剂,可破坏RIPK1介导的信号传导,并可减轻炎症和由此产生的组织损伤。RIPK1是肿瘤坏死因子(TNF)受体通路中的一种信号蛋白,在炎症和细胞死亡中发挥关键作用,以应对组织损伤和病原体识别[1]。
|
| 体内研究 (In Vivo) |
第7天,与安慰剂相比,服用eclitasertib的C反应蛋白与基线的相对变化的几何平均比(点估计[90%置信区间])为0.85(0.49-1.45;p=0.30)。C反应蛋白从基线水平下降50%的中位时间为3天,而eclitasertib与安慰剂相比为5天(p=0.056)。在7分临床症状量表上,达到≥2分改善的中位时间为8天,而依西塔替布组与安慰剂组相比为10天(p=0.38)。与安慰剂相比,eclitasertib的平均无呼吸机/呼吸衰竭天数、基线调整后SpO2/FiO2比率的变化和临床生物标志物显示出一致的数值改善。最常报告的治疗合并不良事件是胃肠道疾病和病情加重/恶化的新冠肺炎肺炎。与安慰剂相比,在严重新冠肺炎患者中,依立沙替布具有良好的耐受性,炎症生物标志物的快速解决和临床改善趋势一致。[1]
|
| 酶活实验 |
SAR443122(eclitasertib)是一种高效、选择性口服RIPK1激酶活性抑制剂,正在开发中,作为皮肤红斑狼疮和溃疡性结肠炎的免疫调节药物。受体相互作用丝氨酸/苏氨酸蛋白激酶1(RIPK1)是一种细胞内蛋白,通过表现出激酶活性依赖性和激酶活性非依赖性功能,调节肿瘤坏死因子受体1(TNFR1)、toll样受体(TLRs)3和4以及干扰素受体(IFNRs)的下游信号传导。RIPK1介导的信号传导促进炎症并诱导凋亡或坏死性细胞死亡。RIPK1激酶驱动的炎症和细胞死亡都是肿瘤坏死因子-α(TNF-α)诱导的全身炎症反应综合征(SIRS)的关键因素。此外,RIPK1激酶抑制可能抑制血管功能障碍、内皮/上皮细胞损伤和加重炎症信号传导。它可以通过抑制肺上皮细胞的炎症激增和坏死来补充抗病毒治疗,预防或减少严重炎症对呼吸功能和其他器官衰竭的影响。由于RIPK1被认为是促炎细胞死亡的主要调节因子,因此假设选择性靶向其激酶活性以减轻晚期严重新冠肺炎中观察到的高炎症状态的毁灭性后遗症。
|
| 动物实验 |
In this Phase 1b, double-blinded, placebo-controlled study (NCT04469621) a total of 82 patients were screened, of whom 68 patients were eligible and randomized (2:1) to receive eclitasertib 600 mg (300 mg twice daily) or placebo up to 14 days. Primary outcome was relative change in C-reactive protein from baseline to Day 7. Time to clinical improvement using 7-point ordinal scale, ventilator/respiratory failure-free days, change in SpO2/FiO2 ratio, and biomarkers of severe COVID-19 were explored.
|
| 参考文献 |
[1]. Immunomodulatory and clinical effects of receptor-interacting protein kinase 1 (RIPK1) inhibitor eclitasertib (SAR443122) in patients with severe COVID-19: a phase 1b, randomized, double-blinded, placebo-controlled study. Respir Res. 2024 Feb 28;25(1):107.
[2]. Compounds, compositions and methods. WO2017136727A2.
|
| 其他信息 |
Eclitasertib is an orally bioavailable, small-molecule inhibitor of receptor-interacting serine/threonine-protein kinase 1 (RIPK1; receptor-interacting protein 1; RIP1), with potential anti-inflammatory and immunomodulatory activities. Upon oral administration, eclitasertib disrupts RIPK1-mediated signaling, and may attenuate inflammation and the resulting tissue damage. RIPK1, a signaling protein in the tumor necrosis factor (TNF) receptor pathway, plays a key role in inflammation and cell death in response to tissue damage and pathogen recognition.
SAR443122 inhibits receptor-interacting serine/threonine-protein kinase 1 (RIPK1). It is currently being investigated against inflammatory diseases such as rheumatoid arthritis and psoriasis, and against hyperinflammatory states in patients with severe COVID-19. Background: Targeting receptor-interacting serine/threonine protein kinase 1 could mitigate the devastating sequelae of the hyperinflammatory state observed in severe cases of COVID-19. This study explored the immunomodulatory and clinical effects of the receptor-interacting serine/threonine protein kinase 1 inhibitor SAR443122 (eclitasertib) in patients with severe COVID-19. Methods: In this Phase 1b, double-blinded, placebo-controlled study (NCT04469621) a total of 82 patients were screened, of whom 68 patients were eligible and randomized (2:1) to receive eclitasertib 600 mg (300 mg twice daily) or placebo up to 14 days. Primary outcome was relative change in C-reactive protein from baseline to Day 7. Time to clinical improvement using 7-point ordinal scale, ventilator/respiratory failure-free days, change in SpO2/FiO2 ratio, and biomarkers of severe COVID-19 were explored. Results: Geometric mean ratio (point estimate [90% confidence interval]) of the relative change from baseline in C-reactive protein with eclitasertib vs. placebo on Day 7 was 0.85 (0.49-1.45; p = 0.30). Median time to 50% decrease in C-reactive protein from baseline was 3 days vs. 5 days (p = 0.056) with eclitasertib vs. placebo. Median time to ≥ 2-point improvement on 7-point clinical symptoms scale was 8 days vs. 10 days with eclitasertib vs. placebo (p = 0.38). Mean ventilator/respiratory failure-free days, change in baseline-adjusted SpO2/FiO2 ratio, and clinical biomarkers showed consistent numerical improvements with eclitasertib vs. placebo. The most frequently reported treatment-emergent adverse events were gastrointestinal disorders and condition aggravated/worsened COVID-19 pneumonia. Conclusions: Eclitasertib was well tolerated with consistent trends toward more rapid resolution of inflammatory biomarkers and clinical improvement in severe COVID-19 patients than placebo.[1] |
| 分子式 |
C19H18N6O3
|
|---|---|
| 分子量 |
378.38462305069
|
| 精确质量 |
378.144
|
| 元素分析 |
C, 60.31; H, 4.80; N, 22.21; O, 12.68
|
| CAS号 |
2125450-76-0
|
| PubChem CID |
130298939
|
| 外观&性状 |
White to light yellow solid
|
| LogP |
1.7
|
| tPSA |
113Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
570
|
| 定义原子立体中心数目 |
1
|
| SMILES |
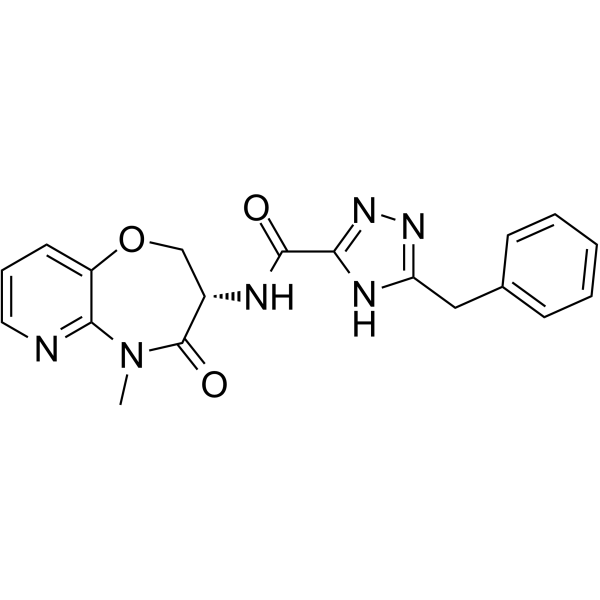
O1C2=CC=CN=C2N(C)C([C@H](C1)NC(C1=NNC(CC2C=CC=CC=2)=N1)=O)=O
|
| InChi Key |
XUZICJHIIJCKQQ-ZDUSSCGKSA-N
|
| InChi Code |
InChI=1S/C19H18N6O3/c1-25-17-14(8-5-9-20-17)28-11-13(19(25)27)21-18(26)16-22-15(23-24-16)10-12-6-3-2-4-7-12/h2-9,13H,10-11H2,1H3,(H,21,26)(H,22,23,24)/t13-/m0/s1
|
| 化学名 |
(S)-3-benzyl-N-(5-methyl-4-oxo-2,3,4,5-tetrahydropyrido[3,2-b][1,4]oxazepin-3-yl)-1H-1,2,4-triazole-5-carboxamide
|
| 别名 |
DNL-758; SAR443122;DNL 758; SAR-443122; DNL758; SAR 443122;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~330.36 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6428 mL | 13.2142 mL | 26.4285 mL | |
| 5 mM | 0.5286 mL | 2.6428 mL | 5.2857 mL | |
| 10 mM | 0.2643 mL | 1.3214 mL | 2.6428 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。