| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
Mps1 (IC50 = 1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Empesertib (BAY1161909) 是一种非常有效的 Mps-1 抑制剂,在 HeLa 细胞增殖测定中显示 IC50 低于 400 nM,在 Mps-1 激酶测定中显示 IC50 低于或等于 1 nM(比 1 nM 更有效)浓度为 1 μM/2 mM ATP。[1]
BAY 1161909和BAY 1217389抑制Mps1激酶活性,IC50值低于10nmol/L,同时显示出优异的选择性。在细胞机制分析中,两种Mps1抑制剂都消除了诺考达唑诱导的SAC活性,并诱导了有丝分裂的过早退出(“有丝分裂突破”),导致多核性和肿瘤细胞死亡。这两种化合物在体外均能有效抑制肿瘤细胞增殖(IC50 nmol/L范围)。[2] 只有相应的R-异构体39-41/Empesertib(BAY1161909)(表6)具有良好的效力;S-异构体42和较大的α-取代基(43,44)导致效力降低(表7)。与氟代氮杂环丁烷衍生的酰胺39和恶唑烷酮40相比,甲基砜41/Empesertib(BAY1161909)显示出最佳的总体特征,与早期先导化合物9相比,进一步提高了激酶选择性(41/Empesertib (BAY1161909)仅抑制两种激酶JNK2和JNK3,在1μmol/L的浓度下超过50%,在230种激酶的Millipore激酶组中,100 nmol/L没有其他激酶),并被选为我们的第一个临床候选物。事实上,它是2014年5月第一个进入I期临床试验的MPS1抑制剂(NCT02138812)[4]。 |
| 体内研究 (In Vivo) |
Empesertib (BAY1161909)是一种极其有效的 Mps-1 抑制剂。通过大鼠肝微粒体测定,其在大鼠体内的最大口服生物利用度(Fmax)大于70%;同样,通过狗肝微粒体测量,其在狗体内的最大口服生物利用度(Fmax)大于50%;根据人体肝微粒体测定,其人体最大口服生物利用度大于 60%。 [1]
在体内,Empesertib (BAY1161909)和BAY 1217389在肿瘤异种移植物研究中的单一疗法中取得了中等疗效。然而,根据其独特的作用方式,当与紫杉醇联合使用时,低剂量的Mps1抑制剂通过削弱SAC活性来减少紫杉醇诱导的有丝分裂阻滞。因此,在广泛的异种移植物模型中,包括那些显示获得性或内在紫杉醇耐药性的模型中,联合治疗在各自的MTD上比紫杉醇或Mps1抑制剂单药治疗有显著提高的疗效。两种Mps1抑制剂均表现出良好的耐受性,且不会增加紫杉醇单一疗法的毒性。这些临床前研究结果验证了癌症治疗中废除SAC的创新概念,并证明了临床概念验证研究的合理性,这些研究评估了Mps1抑制剂BAY 1161909和BAY 1217389与抗有丝分裂癌症药物联合使用,以提高其疗效并潜在地克服耐药性[2]。 |
| 酶活实验 |
Kinase assay [2]
通过基于TR-FRET的体外激酶实验评估了Empesertib (BAY1161909)或BAY 1217389对重组人Mps1的抑制作用,实验中使用了一种生物素化肽(biotin-Ahx-PWDPDDADITEILG-NH2)的磷酸化反应。在酶反应开始前,激酶和测试化合物预孵育15分钟,然后加入底物和10 μmol/L的ATP启动反应。更多细节请参见补充材料和方法。 Kinase selectivity profiling [2] 使用Millipore Kinase或DiscoveRx profiler筛选平台对Empesertib (BAY1161909)和BAY 1217389进行了一系列激酶的反向筛选。Empesertib (BAY1161909)最初在DiscoveRx激酶面板中以1 μmol/L的浓度进行测试,随后对11种激酶进行了KD测定(补充表S1)。BAY 1161909在Millipore激酶面板中以10 μmol/L的浓度进行测试,随后在1和0.1 μmol/L的浓度下重新测试,并对JNK1alpha、JNK2alpha和JNK3进行了IC50测定(补充表S1)。BAY 1217389最初在DiscoveRx激酶面板中以1、0.1和0.01 μmol/L的浓度进行测试(补充表S2)。 Biochemical assays [3] 通过IMAP®荧光偏振实验测定了生化纯化的全长TTK激酶活性的抑制,如前所述。简而言之,激酶抑制剂和TTK在IMAP反应缓冲液中稀释,该缓冲液由10 mM Tris–HCl(pH 7.5)、10 mM MgCl2、0.01% Tween-20、0.1% NaN3和1 mM DTT组成。在室温下避光预孵育1小时后,加入荧光素标记的MBP衍生底物肽,随后加入ATP启动反应。最终酶浓度为3.9 nM,最终底物浓度为50 nM,最终ATP浓度为5 μM。反应在室温下避光进行2小时。荧光偏振在Envision多模式读数器上测量。数据使用XLfit™5中的四参数逻辑曲线拟合。实验至少重复三次(补充表S2)。 Purification of TTK kinase domain [3] TTK根据结构基因组联盟开发的协议进行表达和纯化。使用质粒TTKA-c013/SGC2-B08。TTK在大肠杆菌Rosetta™菌株中表达,培养基为LB,含有50 μg/L卡那霉素和35 μg/L氯霉素,在37 °C摇床培养至OD600为0.6–0.8。之后,加入1 mM IPTG,并在室温下继续孵育4小时。细菌沉淀在−20 °C冷冻。纯化时,细胞重悬于结合缓冲液[50 mM Hepes(pH 7.5)、500 mM NaCl和5%(体积/体积)甘油]。加入10 mM咪唑和无EDTA的蛋白酶抑制剂(每50 mL 1片),并使用Avastin液体均质器裂解细胞,随后离心。上清液与NiNTA Superflow-sepharose珠在4 °C孵育1小时,之后分离珠子并用含50 mM咪唑的结合缓冲液洗涤。蛋白用含250 mM咪唑的结合缓冲液洗脱。第二天,含蛋白的馏分通过Superdex 75柱进行尺寸排阻色谱进一步纯化,运行缓冲液为50 mM Hepes(pH 7.5)、150 mM NaCl和5 mM DTT。峰馏分合并后,在干冰/乙醇中快速冷冻,分装为50 μl,储存于−80 °C。特别用于蛋白质晶体学的峰馏分在4 °C下使用Spin-X UF 30-kDa过滤器浓缩至8–10 mg/ml,然后快速冷冻储存。 Thermal shift assays [3] 将纯化的TTK激酶结构域解冻并在IMAP缓冲液[10 mM Tris(pH 7.5)、10 mM MgCl2、0.01% Tween-20和0.1% NaN3]中稀释至4.8 μM。随后,将10 μl蛋白与5 μl 40 μM的目标化合物在IMAP缓冲液中混合,置于Greiner 96孔PCR板中。在冰上孵育30分钟后,加入5 μl 1250倍稀释的SyproOrange(在IMAP缓冲液中)。最终浓度为2.4 μM TTK和10 μM化合物。SyproOrange稀释5000倍。孵育后立即用TopSeal A-plus密封板,并转移至Biorad CFX96,温度从20 °C升至90 °C,每5秒增加0.5 °C。报告值在三次独立实验中各测量四次(补充表S2)。通过取熔解曲线一阶导数的最小值确定熔解温度。 SPR [3] 使用Biacore T200通过表面等离子体共振(SPR)测定结合动力学。由于使用全长TTK的初始测试未能获得稳定的表面,因此我们使用了His标记的TTK激酶结构域,如前所述。简而言之,TTK激酶结构域通过His标签捕获和胺偶联固定在Ni-NTA传感器芯片上,偶联水平为4000–6000 RU。抑制剂在结合缓冲液中稀释,最终DMSO浓度为1%(体积/体积)。化合物结合在结合缓冲液(10 mM Tris、10 mM MgCl2、0.01% Tween-20和1 mM DTT,pH 6.8)中测量,DMSO浓度为1%(体积/体积),使用单循环动力学,注射浓度范围为1 – 3.16 – 10 – 31.6 – 100 nM。流速为30 μl/min,每次注射的结合时间为100秒。最后一次注射后的解离至少监测30分钟。未进行再生。从化合物信号中减去缓冲液注射和参考通道信号(双参考)。使用Biacore评估软件将结果数据拟合到简单的1:1 Langmuir结合模型。所有动力学常数均在Biacore T200的工作范围内。为了确定曲线拟合的可靠性,我们应用了标准的Biacore检查。拟合的唯一性(U值)、ka和kd的标准误差以及质量转移常数均按之前所述进行监控。 生化MPS1抑制试验。在时间分辨荧光共振能量转移(TR-FRET)的基础上,用磷酸化特异性抗体测定生物素化底物肽的磷酸化,评估了人类MPS1的抑制作用。在20μM ~ 0.1 nM范围内的11种不同浓度下,在同一微滴板上测试化合物,每种浓度重复一次。稀释系列在测定前单独配制为100倍浓度的DMSO原液。在实验中,将每种测试化合物在DMSO中的原液50nL移液到384孔的黑色小体积微滴板中。加入2微升人重组MPS1水溶液[25 mM HEPES pH 7.7, 10 mM MgCl2, 2mM DTT, 0.05% BSA (w/v), 0.1 mM活化的原钒酸钠,0.001% Pluronic F-127],在22℃下孵育15分钟,使测试化合物与酶预结合。然后,加入3μL的ATP溶液(低ATP浓度为16.7μM,高ATP浓度为3.3mM)启动激酶反应;5μL测定体积的终浓度分别为10μM和2mM)和生物素化肽生物素- ahx - pwdpddaditeilg(酰胺型c端)(1.67μM;末浓度=1μM),在22℃下孵育60min。酶的浓度根据酶组的活性进行调整,并选择适当的浓度以使测定在线性范围内(典型浓度在0.25-0.5nM范围内)。在EDTA水溶液(40mM EDTA, 0.1% (w/v) BSA, 100mM HEPES pH 7.4)中加入5μL的TR-FRET检测试剂溶液[140 nM streptavidin-XLent和1.5nM抗磷酸化(Ser/Thr)-europium抗体]停止反应。所得混合物在22℃下孵育1小时,使磷酸化的生物素化肽与检测试剂之间形成络合物。随后,通过测量从铕螯合物到链亲和素- xlent的共振能量转移来评估磷酸化底物的量。为此,使用TR-FRET阅读器[例如,PHERAstar]测量在350nm激发后在620nm和665nm处的荧光发射。以665nm和620nm发射光谱的比值作为测定磷酸化底物量的指标。数据归一化(无抑制剂的酶反应=0%抑制,所有其他检测成分但无酶反应=100%抑制),并使用四参数拟合计算ic50值。结合动力学研究。表面等离子体共振(SPR)光谱在Biacore T100仪器上进行。重组hMPS1激酶结构域(N515-T806)在大肠杆菌含有凝血酶裂解位点的n端gst融合蛋白中表达,通过谷胱甘肽亲和层析纯化,然后进行凝血酶裂解,随后进行尺寸排除层析,如下所述,用于结晶实验的蛋白生产。通过胺偶联将该蛋白固定在S系列CM5传感器芯片上,密度为1000和5000 RU。在测定缓冲液[25mM HEPES pH7.7, 10mM MgCl2, 2mM DTT, 0.001% (v/v)表面活性剂P20, 2% DMSO]中预先平衡固定蛋白后,将5系列稀释的测试化合物应用于多个注射周期(16个:浓度为3.9,7.8,15.6,31.3,62.5,125,250,500和1000 nM)或单次注射周期(41个:浓度为2,7.8,31.3,125和500 nM)。所得到的传感器图拟合到包含质量输运标准项的简单Langmuir 1:1相互作用模型,从中得到动力学参数[kon, koff,(=1/koff)]。[4] 大鼠肝细胞体外代谢稳定性研究。[4] 采用两步灌注法分离雄性Wistar大鼠肝细胞。灌注后,小心地从大鼠身上取出肝脏,打开肝包膜,轻轻摇出肝细胞,放入冰冷的Williams培养基E (WME)培养皿中。将得到的细胞悬液通过无菌纱布过滤到50mL Falcon管中,在50×gfor下离心3min。将细胞颗粒在30mL WME中重悬,在100×g下通过Percoll梯度离心两次。再次用WME洗涤肝细胞,并在含5% FCS的培养基中重悬。台盼蓝法测定细胞活力。为了进行代谢稳定性试验,将肝细胞在含有5% FCS的WME中以1.0 × 106个生命细胞/mL的密度分布到玻璃瓶中。将待测化合物添加至终浓度为1μM。培养中的有机溶剂限制在≤0.01% DMSO和≤1%乙腈。孵育期间,肝细胞悬液以580 rpm连续振荡,在2、8、16、30、45和90 min取等量,立即加入等体积的冷乙腈。样品在-20°C下冷冻过夜,然后在3000rpm下离心15分钟。上清用Agilent 1200高效液相色谱系统进行MS/MS检测。试验化合物的半衰期由浓度-时间图确定。根据半衰期,计算本征清除率和体外预测清除率,并根据“充分搅拌”肝脏模型计算EH=(CLb/LBF)·100%的肝脏提取率。3结合标准化肝血流量(LBF)和体内、体外肝细胞数量,计算体外血清除率(CLb,体外)和肝提取比(EH,体外)。肝血流量:大鼠、狗、人分别为4.2、2.1、1.32L/h/kg;比肝重:大鼠、狗、人分别为32、39、21 g/kg体重;活体肝细胞,1.1×108cells/g肝;体外肝细胞,1.0×106/mL。 肝微粒体体外代谢稳定性研究。[4] 在100mM磷酸盐缓冲液pH7.4 (NaH2PO4·H2O + Na2HPO4·2H2O)中,以1μM浓度的肝微粒体悬液孵育,蛋白浓度0.5 mg/mL, 37℃,测定化合物的体外代谢稳定性。在pH 7.4的磷酸盐缓冲液中加入含有8 mM葡萄糖-6-磷酸、0.5 mM NADP和1 IU/mL葡萄糖-6-磷酸脱氢酶的辅因子,激活微粒体。随后不久,将试验化合物以1mL的终体积加入孵育中,开始代谢测定。孵育期间,以580转/分的速度连续摇匀微粒体悬液,并在2、8、16、30、45和60分钟取等分。按照上述肝细胞法进一步处理和分析。 |
| 细胞实验 |
在 96 孔多滴定板上,将培养的肿瘤细胞以 5000 个细胞/孔(MCF7、DU145、HeLa-MaTu-ADR)、3000 个细胞/孔(NCI-H460、HeLa-MaTu、HeLa)或 1000 个细胞/孔的密度铺板。细胞/孔(B16F10),添加有 10% 胎牛血清的五种生长培养基各 200 μl。”将测试物质添加到其他板中的新鲜培养基(200 μl)中,该培养基已更换为不同的培养基浓度(0 μM,以及0.01-30 μM范围内)。将细胞在测试物质存在下孵育4天。24小时后,将一块板(零点板)上的细胞结晶紫染色,通过结晶紫染色评估细胞增殖情况,固定细胞时,每点加入11%戊二醛溶液20μl,室温静置15分钟,固定细胞15用水洗涤3次,然后在室温下干燥平板,细胞染色包括添加100μl/测量点的0.1%结晶紫溶液(pH 3.0)。对染色细胞进行三轮水洗后,将板在室温下干燥。为了溶解染料,添加 100 μl/测量点的 10% 乙酸溶液。
Multinuclearity化验。[2] 将U-2 OS 细胞以每孔2500个细胞的密度在384孔微滴板中接种于20 μL细胞培养基中,37℃孵育过夜。以终浓度为100 nmol/L,分三次加入Empesertib (BAY1161909)或BAY 1217389。细胞在37℃条件下分别处理0、24、48和72小时。之后,用4% (v/v) PFA固定细胞,0.5% (v/v) Triton X-100渗透细胞,并用0.5% (v/v) BSA在PBS中阻断细胞。抗体标记法检测α-微管蛋白结构。用山羊IgG阻断后,在阻断液中加入二抗。用PBS洗涤细胞,并用Hoechst 33342标记细胞核。最后,将细胞洗净,用PBS覆盖,并在4°C下保存,直到获取图像。使用PerkinElmer Opera高含量分析阅读器获取图像。 细胞分析。[4]< 人类肿瘤细胞系从ATCC或德国微生物和细胞培养物收集中获得。细胞系的鉴定在德国微生物和细胞培养物收集处进行。细胞在建议的生长条件下在37°C的加湿培养箱中繁殖。如前所述进行HeLa和HT29细胞增殖试验。 纺锤体组装检查点(SAC)试验(基于细胞的机制试验)[4]< SAC试验按照我们公布的方案进行。活细胞成像联合治疗研究。对于荧光活细胞成像,实验按照我们公布的方案进行。 |
| 动物实验 |
rat, dog and humans
1-2 mg/mL Oral gavage, IV Pharmacokinetic investigations [2] Pharmacokinetic studies were performed in male Wistar rats and female CD1 or NMRI nu/nu mice. For i.v. studies in rats and mice, Empesertib (BAY1161909) was solubilized in 1% DMSO, 99% plasma; for p.o. studies in rats in 50% polyethylene glycol (PEG) 400, 10% ethanol, 40% water, and for p.o. studies in mice in 75% PEG 400, 5% ethanol, and 25% solutol. BAY 1217389 was solubilized in 50% PEG 400, 10% ethanol, and 40% water for intravenous and p.o. dosing in rats and mice. In pharmacokinetic studies, plasma samples were collected after 2, 5, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after intravenous application and after 8, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after p.o. administration and precipitated with ice-cold acetonitrile (1:5). Supernatants were analyzed via LC/MS-MS. Pharmacokinetic parameters were estimated from the plasma concentration data, e.g., using the log-linear trapezoidal rule for AUC estimation. Maximal plasma concentrations (Cmax) and time thereof (Tmax) were taken directly from the concentration time profiles. Animal efficacy studies [2] For tumor xenograft studies, female athymic NMRI nu/nu mice (Taconic), 50 days old, average body weight 20 to 22 g, were used after an acclimatization period of 14 days. Feeding and drinking was ad libitum 24 hours per day. Human tumor cells derived from exponentially growing cell cultures were resuspended for A2780cis, NCI-H1299, and SUM-149 models in 100% Matrigel to a final concentration of 2 × 107, 3 × 107, or 5 × 107 cells/mL, respectively. Subcutaneous implants of 0.1 mL of 2 × 106 A2780cis, 3 × 106 NCI-H1299, or 5 × 106 SUM-149 cells were inoculated into the inguinal region of athymic mice. Tumor fragments of patient tumor explants MAXF 1384 or LU384, obtained from serial passage in nude mice, were cut into fragments of 4 to 5 mm diameter and transplanted subcutaneously into the flank of athymic mice. Tumor area (product of the longest diameter and its perpendicular), measured with a caliper, and body weight were determined two to three times a week. Tumor growth inhibition is presented as treatment/control ratio (T/C) calculated with tumor areas at the end of the study. Animal body weight was used as a measure for treatment-related toxicity. Body weight loss > 20% was dedicated as toxic. When tumors reached a size of approximately 20 to 40 mm², depending on growth of the tumor model, animals were randomized to treatment and control groups (8–10 mice/group) and treated p.o. with vehicle (70% PEG 400, 5 % ethanol, and 25% Solutol), Empesertib (BAY1161909), BAY 1217389, and/or paclitaxel, as indicated in Tables and Figure legends. In combination treatment groups, Mps1 inhibitor and paclitaxel were applied at the same day within a time frame of 1 hour. The treatment of each animal was based on individual body weight. In vivo mode of action studies [2] For analysis of polyploidy and multinuclearity induction in vivo, A2780cis tumor–bearing female NMRI nude mice (see above) were treated with paclitaxel (intravenously once with 24 mg/kg), Empesertib (BAY1161909) (p.o. twice daily for 2 days with 2.5 mg/kg), and in combination with paclitaxel (i.v. once 24 mg/kg) and Empesertib (BAY1161909) (p.o. twice daily for 2 days 1 mg/kg). Treatment for all groups started at a tumor size of 60 mm² at day 14 after tumor cell inoculation. Tumor samples were prepared 4 and 8 hours after first Empesertib (BAY1161909) application at treatment day 1, as well as 4, 8, and 24 hours after first application of BAY 1161909 on treatment day 2. At each time point, 3 animals per treatment group were analyzed. Tumors were used for histologic examination after paraffin embedding and hematoxylin and eosin staining. In Vivo Pharmacokinetics in Rats and Dogs. [4] All animal experiments were conducted in accordance with the German Animal Welfare Law and were approved by local authorities.For in vivopharmacokinetic experiments, test compounds were administered to male Wistar rats or female Beagle dogs intravenously at doses of 0.1 to 0.5 mg/kg and intragastrically at doses of 0.2 to 1.0 mg/kg formulated as solutions using solubilizers such as PEG400in well-tolerated amounts. Concerning iv administration, test compounds were given as bolus (rat) or 15 min infusion (dog). Blood samples were taken at various time points after dosing, usually up to 24 h. Depending on the expected half-life, additional samples were taken at later time points (e.g., 48h, 7h). Blood was collected into lithium heparin tubes and centrifuged at 3000rpm for 15min. An aliquot of 100μL from the supernatant (plasma) was taken and precipitated by the addition of 400μL of cold acetonitrile. Samples were frozen at –20°C overnight, and subsequently thawed and centrifuged at 3000rpm, 4°C for 20 S8min. Aliquots of the supernatants were analyzed using an Agilent 1200 HPLC system with MS/MS detection. PK parameters such as AUC and CL were calculated by non-compartmental analysis using PK calculation software. Animal [4] For tumor xenograft studies, female athymic NMRI nu/nu mice (Taconic), 50 days old, average body weight 20–22 g, were used after an acclimatization period of 14 days. Feeding and drinking was ad libitum 24 hours per day. Human NCI-H1299 tumor cells derived from exponentially growing cell cultures were resuspended in 100% Matrigel to a final concentration of 3×107cells/mL and implanted subcutaneously in 0.1mL into the inguinal region of athymic NMRI nude mice. Tumor fragments of patient tumor explants MAXF 1384, obtained from serial passage in nude mice, were cut into fragments of 4–5mm diameter and transplanted subcutaneously into the flank of athymic NMRI nude mice. Tumor area (product of the longest diameter and its perpendicular), measured with a caliper, and body weight were determined 2–3 times per week. Tumor growth inhibition is presented as the treatment/control ratio (T/C), calculated with tumor areas at the end of the study. Animal body weight was used as a measure of treatment-related toxicity. Body weight loss >20% was considered toxic. When tumors reached a size of approximately 20–40 mm2, animals were randomized into treatment and control groups (8–10 mice/group), S9and treated twice daily for 2 days on and 5 days off orally (po) with vehicle (70% PEG 400, 5% EtOH, 25% Solutol), 41(Empesertib (BAY1161909)), 79(BAY 1217389), and/or once per week intravenously (iv) with paclitaxel, as indicated in figure 8. In combination treatment groups, MPS1 inhibitor and paclitaxel were applied on the same day within a time frame of 1 h. The treatment of each animal was based on individual body weight. Animals were euthanized according to the German Animal Welfare Guidelines. Data are expressed as tumor area means ± SD. Statistical analysis included one-way ANOVA and differences were compared versus the control group by a pairwise comparison procedure using SigmaStat software |
| 药代性质 (ADME/PK) |
In vivo pharmacokinetic parameters [2]
Pharmacokinetic parameters were determined in mouse and rat. Following intravenous administration of BAY 1161909 as bolus of 0.5 mg/kg to male CD1 mouse and 0.5 mg/kg to male Wistar rat, the compound exhibited low blood clearance. The volume of distribution (Vss) was high in both species and terminal half-lives were long. After oral administration of 1 mg/kg to female NMRI mouse and 0.5 mg/kg to male Wistar rat, peak plasma levels were reached after 4 hours. The oral bioavailability was moderate in mouse and rat (Table 1). BAY 1217389 was administered intravenously as bolus of 1.0 and 0.5 mg/kg to female CD1 mouse and male Wistar rat, respectively. Blood clearance was found to be low in the tested species. Vss was high and terminal half-lives were long. BAY 1217389 was administered orally to female NMRI mouse (1 mg/kg) and male Wistar rat (0.5 mg/kg). Peak plasma concentrations were observed between 1.5 and 7 hours. Oral bioavailability was high in rat and moderate in mouse (Table 1). Our data demonstrate that we have identified two novel Mps1 kinase inhibitors with a favorable pharmacokinetic profile, supporting further development for clinical application. |
| 参考文献 | |
| 其他信息 |
Empesertib is an orally bioavailable, selective inhibitor of the serine/threonine monopolar spindle 1 (Mps1) kinase, with potential antineoplastic activity. Upon administration, empesertib binds to and inhibits the activity of Mps1. This causes inactivation of the spindle assembly checkpoint (SAC), accelerated mitosis, chromosomal misalignment, chromosomal missegregation, mitotic checkpoint complex destabilization, and increased aneuploidy. This leads to the induction of cell death in cancer cells overexpressing Mps1. Mps1, a kinase expressed in proliferating normal tissues and aberrantly overexpressed in a wide range of human tumors, is activated during mitosis and is essential for SAC functioning and controls chromosome alignment.
Monopolar spindle 1 (Mps1) has been shown to function as the key kinase that activates the spindle assembly checkpoint (SAC) to secure proper distribution of chromosomes to daughter cells. Here, we report the structure and functional characterization of two novel selective Mps1 inhibitors, BAY 1161909 and BAY 1217389, derived from structurally distinct chemical classes. BAY 1161909 and BAY 1217389 inhibited Mps1 kinase activity with IC50 values below 10 nmol/L while showing an excellent selectivity profile. In cellular mechanistic assays, both Mps1 inhibitors abrogated nocodazole-induced SAC activity and induced premature exit from mitosis ("mitotic breakthrough"), resulting in multinuclearity and tumor cell death. Both compounds efficiently inhibited tumor cell proliferation in vitro (IC50 nmol/L range). In vivo, BAY 1161909 and BAY 1217389 achieved moderate efficacy in monotherapy in tumor xenograft studies. However, in line with its unique mode of action, when combined with paclitaxel, low doses of Mps1 inhibitor reduced paclitaxel-induced mitotic arrest by the weakening of SAC activity. As a result, combination therapy strongly improved efficacy over paclitaxel or Mps1 inhibitor monotreatment at the respective MTDs in a broad range of xenograft models, including those showing acquired or intrinsic paclitaxel resistance. Both Mps1 inhibitors showed good tolerability without adding toxicity to paclitaxel monotherapy. These preclinical findings validate the innovative concept of SAC abrogation for cancer therapy and justify clinical proof-of-concept studies evaluating the Mps1 inhibitors BAY 1161909 and BAY 1217389 in combination with antimitotic cancer drugs to enhance their efficacy and potentially overcome resistance. [2] The protein kinase threonine tyrosine kinase (TTK; also known as Mps1) is a critical component of the spindle assembly checkpoint and a promising drug target for the treatment of aggressive cancers, such as triple negative breast cancer. While the first TTK inhibitors have entered clinical trials, little is known about how the inhibition of TTK with small-molecule compounds affects cellular activity. We studied the selective TTK inhibitor NTRC 0066-0, which was developed in our own laboratory, together with 11 TTK inhibitors developed by other companies, including Mps-BAY2b, BAY 1161909, BAY 1217389 (Bayer), TC-Mps1-12 (Shionogi), and MPI-0479605 (Myrexis). Parallel testing shows that the cellular activity of these TTK inhibitors correlates with their binding affinity to TTK and, more strongly, with target residence time. TTK inhibitors are therefore an example where target residence time determines activity in in vitro cellular assays. X-ray structures and thermal stability experiments reveal that the most potent compounds induce a shift of the glycine-rich loop as a result of binding to the catalytic lysine at position 553. This "lysine trap" disrupts the catalytic machinery. Based on these insights, we developed TTK inhibitors, based on a (5,6-dihydro)pyrimido[4,5-e]indolizine scaffold, with longer target residence times, which further exploit an allosteric pocket surrounding Lys553. Their binding mode is new for kinase inhibitors and can be classified as hybrid Type I/Type III. These inhibitors have very potent anti-proliferative activity that rivals classic cytotoxic therapy. Our findings will open up new avenues for more applications for TTK inhibitors in cancer treatment. [3] Inhibition of monopolar spindle 1 (MPS1) kinase represents a novel approach to cancer treatment: instead of arresting the cell cycle in tumor cells, cells are driven into mitosis irrespective of DNA damage and unattached/misattached chromosomes, resulting in aneuploidy and cell death. Starting points for our optimization efforts with the goal to identify MPS1 inhibitors were two HTS hits from the distinct chemical series "triazolopyridines" and "imidazopyrazines". The major initial issue of the triazolopyridine series was the moderate potency of the HTS hits. The imidazopyrazine series displayed more than 10-fold higher potencies; however, in the early project phase, this series suffered from poor metabolic stability. Here, we outline the evolution of the two hit series to clinical candidates BAY 1161909 and BAY 1217389 and reveal how both clinical candidates bind to the ATP site of MPS1 kinase, while addressing different pockets utilizing different binding interactions, along with their synthesis and preclinical characterization in selected in vivo efficacy models.[4] |
| 分子式 |
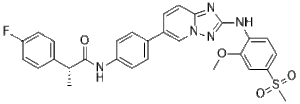
C29H26FN5O4S
|
|---|---|
| 分子量 |
559.61
|
| 精确质量 |
559.168
|
| 元素分析 |
C, 62.24; H, 4.68; F, 3.39; N, 12.51; O, 11.44; S, 5.73
|
| CAS号 |
1443763-60-7
|
| 相关CAS号 |
1443763-60-7;2170218-39-8;2170218-40-1 (tosylate hydrate);
|
| PubChem CID |
71599640
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.658
|
| LogP |
4.44
|
| tPSA |
123
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
951
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1(F)=CC=C(C=C1)[C@@H](C)C(NC1C=CC(C2C=CC3N(C=2)N=C(NC2C=CC(S(C)(=O)=O)=CC=2OC)N=3)=CC=1)=O
|
| InChi Key |
NRJKIOCCERLIDG-GOSISDBHSA-N
|
| InChi Code |
InChI=1S/C29H26FN5O4S/c1-18(19-4-9-22(30)10-5-19)28(36)31-23-11-6-20(7-12-23)21-8-15-27-33-29(34-35(27)17-21)32-25-14-13-24(40(3,37)38)16-26(25)39-2/h4-18H,1-3H3,(H,31,36)(H,32,34)/t18-/m1/s1
|
| 化学名 |
(2R)-2-(4-fluorophenyl)-N-[4-[2-(2-methoxy-4-methylsulfonylanilino)-[1,2,4]triazolo[1,5-a]pyridin-6-yl]phenyl]propanamide
|
| 别名 |
Empesertib; Mps1-IN-5; BAY1161909; 1443763-60-7; Mps1-IN5; BAY 1161,909; BAY-1161,909; BAY1161,909; Empesertib [INN]; Empesertib [WHO-DD]; BAY-1161909; BAY 1161909
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 35 mg/mL (~62.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.47 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (4.47 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.47 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7870 mL | 8.9348 mL | 17.8696 mL | |
| 5 mM | 0.3574 mL | 1.7870 mL | 3.5739 mL | |
| 10 mM | 0.1787 mL | 0.8935 mL | 1.7870 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。