| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
HDAC1 ( IC50 = 243 nM ); HDAC3 ( IC50 = 248 nM ); HDAC2 ( IC50 = 453 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:MS-275 通过 2-氨基显示出对 HDAC 的抑制作用。 MS-275 诱导 K562 细胞中 p21WAF1/CIP1 和凝溶胶蛋白的积累。 MS-275可以减少A2780细胞中的S期细胞并诱导G1期细胞。 MS-275 抑制人类肿瘤细胞系的增殖,包括 A2780、Calu-3、HL-60、K562、St-4、HT-29、KB-3-1、Capan-1、4-1St 和 HCT-15 IC50 从 41.5 nM 至 4.71 μM,这是由于 HAD 抑制所致。 MS-275 对其他 HDAC(4、6、8 和 10)不敏感,IC50 约为/高于 100 μM。 MS-275 对人类白血病和淋巴瘤细胞(包括 U937、HL-60、K562 和 Jurkat)显示出极大的抑制作用。 MS-275 还降低细胞周期蛋白 D1 以及抗凋亡蛋白 Mcl-1 和 XIAP 的表达。激酶测定:用 HDAC 缓冲液按 1:6 稀释大鼠肝酶。重组人 HDAC 在 HDAC 缓冲液中按 1:4 稀释。对于标准 HDAC 测定,将 60 μL HDAC 缓冲液与 10 μL 稀释酶溶液在 30 °C 下混合。 HDAC 反应通过在 HDAC 缓冲液中添加 30 μL 底物溶液开始,然后在 30 °C 下孵育 30 分钟。添加 100 μL 胰蛋白酶溶液(10 mg/ml 胰蛋白酶于 50 mM Tris-HCl [pH 8.0]、100 mM NaCl、2 μM TSA 中)终止反应。在 30°C 下孵育 20 分钟后,通过测量 460 nm(λex = 390 nm)处的荧光来监测 AMC 的释放。使用免费 AMC 校准荧光强度。对于标准时程实验,在初始 100 μL HDAC 反应中使用 20 pmol 底物。 Km 和 Vmax 值通过测量 2-50 pmol 底物酶裂解产生的荧光 AMC 来确定。使用哈内斯图分析实验数据。 AMC 信号是针对含有缓冲液和底物但不含酶的空白记录的。细胞检测:癌细胞(A2780、Calu-3、HL-60、K562、St-4、HT-29、KB-3-1、Capan-1、4-1St 和 HCT-15 细胞,5 × 103)接种到96孔板的每个孔中并用分级浓度的MS-275培养3天。将细胞用 0.1 mg/mL 中性红在 CO2 培养箱中染色 1 小时,吸出培养基后,测量用 50 μL 乙醇和 150 μL 0.1 M Na2HPO4 溶解的中性红的 OD540。 IC50值是通过将细胞的生长抑制与药物浓度的对数作图来确定的。
|
| 体内研究 (In Vivo) |
MS-275 在 49 mg/kg 浓度下对除 HCT-15 之外的人类肿瘤异种移植物表现出良好的抗肿瘤活性。 MS-275 在实体瘤和血液恶性肿瘤以及生理和异常基因表达的调节方面表现出有希望的治疗潜力。 MS-275与IL-2结合,对因T调节细胞减少和脾细胞增加而导致的肾细胞癌异种移植模型具有良好的抗肿瘤活性。
研究了合成苯甲酰胺衍生物抑制组蛋白去乙酰化酶(HDA)的能力。本研究考察了一种最具活性的苯甲酰胺衍生物MS-27-275的生物学特性和抗肿瘤功效。MS-27-275抑制部分纯化的人HDA,引起多种肿瘤细胞系核组蛋白的超乙酰化。它的行为方式类似于其他HDA抑制剂,如丁酸钠和曲古霉素a;MS-27-275诱导p21(WAF1/CIP1)和gelsolin,改变细胞周期分布,s期细胞减少,g1期细胞增加。MS-27-275对多种人类肿瘤细胞系的体外敏感性谱显示出与常用抗肿瘤药物5-氟尿嘧啶不同的模式,而且有趣的是,p21(WAF1/CIP1)在MS-27-275敏感的细胞系中积累更快、更大。口服MS-27-275对移植到裸鼠体内的8种肿瘤系中的7种有强烈的抑制作用,尽管其中大多数对5-氟尿嘧啶没有反应。一种结构与MS-27-275相似的化合物,没有抑制hda的活性,在细胞培养中既没有生物学效应,也没有体内治疗效果。这些结果表明MS-27-275通过抑制HDA发挥抗肿瘤作用,可能为传统抗肿瘤药物不敏感的癌症提供新的化疗策略。[3] 实验性自身免疫性神经炎(EAN)是一种T细胞介导的周围神经系统自身免疫性炎症性脱髓鞘疾病,是人类炎症性脱髓鞘性多根神经病变的动物模型。MS-275是一种有效的组蛋白去乙酰化酶抑制剂,目前正在进行各种恶性肿瘤的临床研究,据报道显示出有希望的抗炎活性。在我们目前的研究中,从首次神经症状出现开始,每天给EAN大鼠一次MS-275 (3.5 mg/kg i.p),大大降低了EAN的严重程度和持续时间,并减轻了巨噬细胞、T细胞和B细胞的局部积聚,以及坐骨神经脱髓鞘。此外,MS-275处理的EAN大鼠坐骨神经中促炎白介素-1 β、干扰素- γ、白介素-17、诱导型一氧化氮合酶和基质金属蛋白酶-9的mRNA水平显著降低。在淋巴结中,MS-275也抑制促炎细胞因子,但增加抗炎细胞因子白介素-10和Foxp3的表达,Foxp3是调节性T细胞的一种独特的转录因子。此外,MS-275处理增加了EAN大鼠坐骨神经中浸润的Foxp3(+)细胞和抗炎M2巨噬细胞的比例。综上所述,我们的数据表明MS-275可以通过抑制炎性T细胞、巨噬细胞和细胞因子,诱导抗炎免疫细胞和分子,有效抑制EAN中的炎症,提示MS-275是治疗自身免疫性神经病变的有效候选药物[4]。 |
| 酶活实验 |
HDAC 活性生化测定由 Nanosyn 在 384 孔微孔板中进行,反应体积为 10 μL。将 5 微升 2× HDAC 抑制剂(例如 Entinostat)、4 微升 2.5× 酶和 1 微升 10× 底物与测定缓冲液(100 mM HEPES,pH 7.5,25 mM KCl,0.1% BSA,0.01 % Triton X-100、1% DMSO)在典型的酶促反应中。在酶测定中,每种 HDAC 的终浓度范围为 0.5 至 5 nM。在每个实验中,使用的最终底物浓度为 1 μM FAM-RHKK(Ac)-NH2 或 FAM-RHKK(三氟乙酰基)-NH2,并且发现低于每种酶的计算 Km,app[1]。
酶促HDAC活性测定[1] Nanosyn在384孔微孔板中以10μl的反应体积进行HDAC活性的生化测定。标准酶反应在测定缓冲液(100 mm HEPES,pH 7.5,25 mm KCl,0.1%BSA,0.01%Triton X-100,1%DMSO)中含有5μl 2×HDAC抑制剂、4μl 2.5×酶和1μl 10×底物。酶测定中所有HDAC的最终浓度在0.5至5nm之间。在所有测定中使用1μm FAM-RHKK(Ac)-NH2或FAM-RHKK(三氟乙酰基)-NH2的最终底物浓度,发现其低于每种酶的测定Km,app。所有抑制剂在DMSO中连续稀释,然后在测定缓冲液中交叉稀释,并在加入底物引发反应之前与酶一起孵育15分钟。孵育3小时后,通过分别加入EDTA和SDS至终浓度分别为24mm和0.04%来终止反应。使用在Caliper LC3000®上运行的12吸管微流控芯片分离每个反应中的产物和底物。分离条件使用的下游电压为-800V,上游电压为-3000V,筛选压力为-1.4ps.i。产物和底物的荧光在488nm激发,在530nm检测。使用HTS Well Analyzer软件从电泳图计算底物转化率。 组蛋白脱乙酰酶测定。[3] HDA按照Yoshida等人的描述进行了部分纯化,并进行了轻微的修改。K562细胞(2.5×108)在15ml HDA缓冲液(15mM磷酸钾,pH 7.5/5%甘油/0.2mM EDTA)中被破坏。通过离心(35000×g,10分钟)收集细胞核,并将其重新悬浮在含有1M(NH4)2SO4的15ml HDA缓冲液中。经过超声波处理以降低粘度后,通过离心收集上清液,向上清液中加入固体(NH4)2SO4,使最终浓度达到3.5 M,并在0°C下搅拌1小时。通过离心收集的沉淀物再次用4ml HDA缓冲液溶解,并用2升HDA缓冲溶液透析。将透析液装载到用HDA缓冲液平衡的MonoQ HR5/5(Amersham Pharmacia)上,用30ml HDA缓冲溶液中0-1M NaCl的线性梯度洗脱蛋白质。HDA活性的单峰在0.4 M NaCl下洗脱,该部分在-80°C下储存直至使用。通过将K562细胞(108个细胞)在含有0.5 mCi/ml[3H]乙酸钠(152.8 GBq/mmol;NEN)和5 mM NaBu的25 ml生长培养基中在37°C下孵育1小时来标记核组蛋白。按所述提取组蛋白 在含有2μl上述HDA组分、100μg/ml[3H]乙酰化组蛋白和5μl化合物的50μl反应混合物中,在37°C下溶解在HDA缓冲液中10分钟,评估化合物的HDA抑制活性。用50μl 1M HCl和0.55ml乙酸乙酯提取反应释放的[3H]乙酸,并通过液体闪烁计数测量溶剂层中的放射性。为了评估体内HDA抑制作用,提取细胞组蛋白,用酸/尿素/Triton X-100 PAGE检查,然后用考马斯亮蓝R-250染色,如所述。 |
| 细胞实验 |
SH-SY5Y细胞每周分裂两次,并在标准培养条件下保存在37°C、5% CO2的湿润培养箱中。将细胞以每孔 2500 个细胞的密度接种在 20 μL 体积的补充有 10% FBS 的 DMEM/F-12 培养基中后,使细胞在黑色 384 孔板中粘附整夜。第二天在 100% DMSO 中连续稀释后,HDAC 抑制剂(如恩替司他)交叉稀释到培养基中。为了达到所需的抑制剂终浓度(例如,0.1% DMSO),将在培养基中稀释的 5 μL 化合物(例如,恩替司他)添加到细胞板的适当孔中。将处理的细胞在标准组织培养条件下孵育 6、24、48、72 或 96 小时后,使用 CellTiter-Glo 试剂对细胞 ATP 水平进行定量。类似地,与HDAC抑制剂(例如Entinostat)孵育6小时后吸出来自不同细胞板的培养基,并再次用不含抑制剂的培养基洗涤细胞。孵育 24、48、72 或 96 小时后,向细胞添加 25 μL 补充有 10% FBS 和 0.1% DMSO(无抑制剂)的培养基,并使用 CellTiter-Glo 测量细胞 ATP 水平。使用计数时间为 0.1 秒的 Envision 仪器来测量每个时间点的光度[1]。
|
| 动物实验 |
Mice: Subcutaneous injection of A2780 cells (9×106) in PBS suspension is administered subcutaneously into the flank of a naked mouse. For the remaining tumor lines, KB-3-1, HCT-15, 4-1St, Calu-3, St-4, Capan-1, and HT-29, the tumors are passaged multiple times prior to initiating in vivo antitumor testing. A trocar needle is used to implant a tumor lump, measuring 2-3 mm in diameter, subcutaneously into the flank of a nude mouse. Once the tumors are confirmed to have grown in the body (tumor size, 20-100 mm3), treatment with the drugs is initiated in four or five mice per experimental group. For four weeks, one oral dose of entinostat is given five days a week. Tumor width and length are measured twice a week, and the volume of the tumor is computed.
Rats: Male Lewis rats (weight: 170-200 g, 8-10 weeks) are kept in a 12-hour light/dark cycle with unrestricted access to food and drink. Six rats per group receive an intraperitoneal injection of EntinostatMS-275 (3.5 mg/kg) every day from day 10 to day 14 as part of a therapeutic treatment. EntinostatMS-275 is dissolved in phosphate buffered saline (PBS) for injection, and control rats are administered the same volume (1 mL) of PBS. In Vivo Antitumor Activity. [3] A2780 cells (9 × 106) grown in vitro were suspended in PBS and were injected subcutaneously into the flank of nude mouse. For the other tumor lines, KB-3-1, HCT-15, 4-1St, Calu-3, St-4, Capan-1, and HT-29, tumors were passaged several times before starting in vivo antitumor testing, and a tumor lump (2–3 mm in diameter) was transplanted subcutaneously into the flank of a nude mouse by using a trocar needle. Treatment (four or five mice in each experimental group) with the drugs was started after the tumors were confirmed to have grown in the body (tumor size, 20–100 mm3). Entinostat/MS-27-275 and compound 2, both dissolved with 0.05 N HCl, 0.1% Tween 80, and 5-fluorouracil (5-FU) and diluted with physiological saline, were administered orally once daily 5 days per week for 4 weeks. Tumor length and width were monitored twice weekly, and tumor volume was calculated as described. EAN induction and Entinostat/MS-275 treatment [4] EAN was induced as described (Zhang et al., 2008a). Briefly, rats were immunized by s.c. injection at the basal part of tails with 100 μL of an inoculum containing 100 μg of synthetic neuritogenic P2 peptide 57–81. Neurological scores of EAN were evaluated every day as follows: 0=normal, 1=reduced tonus of tail, 2=limp tail, impaired righting, 3=absent righting, 4=gait ataxia, 5=mild paresis of the hind limbs, 6=moderate paraparesis, 7=severe paraparesis or paraplegia of the hind limbs, 8=tetraparesis, 9=moribund, and 10=death. For therapeutic treatment, EAN rats received i.p. injection of Entinostat/MS-275 (3.5 mg/kg) daily from day 10 to day 14 (six rats/group). For injection, EntinostatMS-275 was suspended in phosphate buffered saline (PBS) and the same volume (1 ml) of PBS was given to control rats. |
| 药代性质 (ADME/PK) |
A summary of the pharmacokinetics of MS-275 is presented in Table 5. In part 1, with every other week dosing, the maximum plasma concentrations (Cmax) and the early part of the AUC were poorly characterized, because the first sampling point was taken after tmax. A plasma concentration × time curve following single doses of 2, 4, and 6 mg/m2 is shown in Fig. 1.
On all dosing schedules, maximum plasma levels of MS-275 were reached within 1 h after single and repeated dose administration. Mean Cmax after the first administration increased almost dose-proportionally. MS-275 plasma concentrations declined to ~4% of Cmax within 4 h after single administration in parts 2 and 3 of the study, where the Cmax was well defined, indicating a rapid distribution into tissues. This initial distribution phase was followed by a slower secondary disposition phase. However, terminal half-life values could not be determined appropriately in most patients, because the perceivable linear part of the curve was <2 half-lives. Thus, the calculated terminal half-life values, as well as AUCand apparent clearance values, were only estimated and may be further considered as approximate reference values. The terminal half-life values were estimated to range between 60 and 150 h, irrespective of the dose or schedule, when the perceivable linear part of the curve could be followed for a relatively long time (up to ~168 h post-dose). The accumulation of the drug in plasma following once-weekly and twice-weekly dosing schedules was evaluated to a limited extent due to deviations from the dosing schedule such as skipped doses and dose reductions due to toxicity. Based on the limited data, plasma drug concentrations appeared to increase continuously up to the last dose in cycle 1, indicating that steady state was not achieved in the twice-weekly treatment cohort. There was no clear indication of drug accumulation following once-weekly treatment. https://pubmed.ncbi.nlm.nih.gov/18579665/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Hematologic toxicity and laboratory abnormalities [https://pubmed.ncbi.nlm.nih.gov/18579665/]
The numbers of cycles with hematologic toxicities are listed in Table 3. Overall, hematologic toxicities were rare and none was dose limiting. Grade 3 hemoglobin occurred in 5 of 149 cycles (3%): 1 on the 2 mg/m2 twice-weekly schedule and 4 in the 5 mg/m2 weekly cohort. Grade 3 or 4 neutropenia was seen in 6 of 149 cycles (4%): 2 at 2 mg/m2 and 1 at 4 mg/m2 every other week and in 3 on the 5 mg/m2 weekly schedule. No grade 3 or 4 thrombocytopenia was observed. There was no relationship between the development of myelosuppression and prior chemotherapy or radiation therapy, and none of the episodes was complicated by fever, serious infection, or bleeding. Nonhematologic toxicity [https://pubmed.ncbi.nlm.nih.gov/18579665/] Nonhematologic toxicity is shown in Table 4. The most common adverse events were nausea and asthenia [occurring in 48 and 47 of 149 cycles (32%), respectively]. The second most common event, anorexia, occurred in 23 of 149 cycles (15%). The most common grade 3 or 4 event was asthenia, occurring in 7 of 149 cycles (5%). Asymptomatic hypophosphatemia was common and constituted DLT in two patients. The hypophosphatemia was not associated with other toxicities such as renal insufficiency or other electrolyte imbalances, and no serious complications were observed. One patient with metastatic colon cancer also experienced concurrent grade 3 hyponatremia during an episode of massive abdominal ascites and dehydration associated with rapid tumor progression and decreased oral intake. This patient had no prior history of renal disease, electrolyte wasting, or prior chemotherapy treatment known to contribute to electrolyte wasting. This patient's dehydration and electrolyte abnormalities resolved within 72 h of therapeutic paracentesis, i.v. fluid, and colloid administration. Patients who had grade ≥2 hypophosphatemia underwent analysis of urine and serum electrolytes, were given daily oral phosphorus supplements, and were monitored at least weekly until stabilization of serum phosphorus levels to within normal range. With other HDAC inhibitors, including the hydroxamic acid derivatives, there has been concern for cardiac rhythm disturbances and myocardial infarction, including prolonged QTc intervals and T- and ST-wave abnormalities, particularly based preclinical models. In this study, electrocardiograms were required at baseline, through cycle 1, at the beginning of cycle 2, and at the end of study participation for the every other week dosing and at baseline and as clinically indicated on the twice-weekly and weekly schedules. MUGA scans were done at baseline, before cycles 2 and 4, and every 6 weeks after cycle 4 on the every other week schedule and at baseline and as clinically indicated in the twice-weekly and weekly treatment groups. Eighteen patients had electrocardiograms after the baseline evaluation: 10 on every other week dosing, 3 on twice-weekly dosing, and 5 on weekly dosing schedules. Ten patients completed serial MUGA scanning: 6 on every other week dosing and 2 each on twice-weekly and weekly dosing schedules. No significant electrocardiograms or MUGA abnormalities attributed at least possibly related to MS-275 were noted. |
| 参考文献 |
|
| 其他信息 |
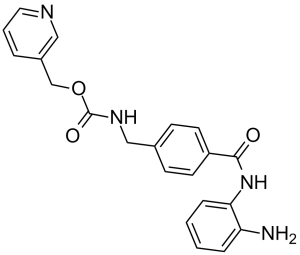
Entinostat is a member of the class of benzamides resulting from the formal condensation of the carboxy group of the pyridin-3-ylmethyl carbamate derivative of p-(aminomethyl)benzoic acid with one of the amino groups of benzene-1,2-diamine. It is an inhibitor of histone deacetylase isoform 1 (HDAC1) and isoform 3 (HDAC3). It has a role as an EC 3.5.1.98 (histone deacetylase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of pyridines, a carbamate ester, a substituted aniline, a primary amino compound and a member of benzamides. It is functionally related to a 1,2-phenylenediamine.
Entinostat is under investigation for the treatment and other of Volunteers, Breast Cancer, Human Volunteers, and Normal Volunteers. Entinostat has been investigated for the treatment of Non-Small Lung Cancer, Epigenetic Therapy. Entinostat is a synthetic benzamide derivative with potential antineoplastic activity. Entinostat binds to and inhibits histone deacetylase, an enzyme that regulates chromatin structure and gene transcription. This agent appears to exert dose-dependent effects in human leukemia cells including cyclin-dependent kinase inhibitor 1A (p21/CIP1/WAF1)-dependent growth arrest and differentiation at low drug concentrations; a marked induction of reactive oxygen species (ROS); mitochondrial damage; caspase activation; and, at higher concentrations, apoptosis. In normal cells, cyclin-dependent kinase inhibitor 1A expression has been associated with cell-cycle exit and differentiation. Effects of the histone deacetylase (HDAC) inhibitor MS-275 have been examined in human leukemia and lymphoma cells (U937, HL-60, K562, and Jurkat) as well as in primary acute myelogenous leukemia blasts in relation to differentiation and apoptosis. MS-275 displayed dose-dependent effects in each of the cell lines. When administered at a low concentration (e.g., 1 micro M), MS-275 exhibited potent antiproliferative activity, inducing p21(CIP1/WAF1)-mediated growth arrest and expression of differentiation markers (CD11b) in U937 cells. These events were accompanied by an increase in hypophosphorylated retinoblastoma protein and down-regulation of cell cycle-related proteins including cyclin D1. However, at higher concentrations (e.g., 5 micro M), MS-275 potently induced cell death, triggering apoptosis in approximately 70% of cells at 48 h. In contrast to other HDAC inhibitors such as apicidin, the extrinsic, receptor-mediated pathway played a minimal role in MS-275 lethality. However, MS-275 potently induced a very early (e.g., within 2 h) increase in reactive oxygen species (ROS), followed by the loss of mitochondrial membrane potential (Delta psi(m)) and cytosolic release of cytochrome c. These events culminated in activation of the caspase cascade, manifested by poly(ADP-ribose) polymerase, p21(CIP1/WAF1), p27(KIP), Bcl-2, and retinoblastoma protein degradation. MS-275 exposure also resulted in diminished expression of cyclin D1 and the antiapoptotic proteins Mcl-1 and XIAP. Administration of the free radical scavenger L-N-acetylcysteine blocked MS-275-mediated mitochondrial injury and apoptosis, suggesting a primary role for ROS generation in MS-275-associated lethality. Lastly, U937 cells stably expressing a p21(CIP1/WAF1) antisense construct were significantly more sensitive to MS-275-mediated apoptosis than controls, but they were impaired in their differentiation response. Together, these findings demonstrate that MS-275 exerts dose-dependent effects in human leukemia cells, i.e., p21(CIP1/WAF1)-dependent growth arrest and differentiation at low drug concentrations and a marked induction of ROS, mitochondrial damage, caspase activation, and apoptosis at higher concentrations.[2] Synthetic benzamide derivatives were investigated for their ability to inhibit histone deacetylase (HDA). In this study, one of the most active benzamide derivatives, MS-27-275, was examined with regard to its biological properties and antitumor efficacy. MS-27-275 inhibited partially purified human HDA and caused hyperacetylation of nuclear histones in various tumor cell lines. It behaved in a manner similar to other HDA inhibitors, such as sodium butyrate and trichostatin A; MS-27-275 induced p21(WAF1/CIP1) and gelsolin and changed the cell cycle distribution, decrease of S-phase cells, and increase of G1-phase cells. The in vitro sensitivity spectrum of MS-27-275 against various human tumor cell lines showed a pattern different than that of a commonly used antitumor agent, 5-fluorouracil, and, of interest, the accumulation of p21(WAF1/CIP1) tended to be faster and greater in the cell lines sensitive to MS-27-275. MS-27-275 administered orally strongly inhibited the growth in seven of eight tumor lines implanted into nude mice, although most of these did not respond to 5-fluorouracil. A structurally analogous compound to MS-27-275 without HDA-inhibiting activity showed neither the biological effects in cell culture nor the in vivo therapeutic efficacy. These results suggest that MS-27-275 acts as an antitumor agent through HDA inhibition and may provide a novel chemotherapeutic strategy for cancers insensitive to traditional antitumor agents.[3] |
| 分子式 |
C21H20N4O3
|
|
|---|---|---|
| 分子量 |
376.41
|
|
| 精确质量 |
376.153
|
|
| 元素分析 |
C, 67.01; H, 5.36; N, 14.88; O, 12.75
|
|
| CAS号 |
209783-80-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
4261
|
|
| 外观&性状 |
White off white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
566.7±50.0 °C at 760 mmHg
|
|
| 熔点 |
159-160ºC
|
|
| 闪点 |
296.6±30.1 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.672
|
|
| LogP |
1.46
|
|
| tPSA |
106.34
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
508
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(NCC1=CC=C(C=C1)C(NC2=CC=CC=C2N)=O)OCC3=CC=CN=C3
|
|
| InChi Key |
INVTYAOGFAGBOE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26)
|
|
| 化学名 |
pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.64 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.53 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (5.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,你可以将100 μL 20.8 mg/mL澄清的DMSO储备液加入到900 μL玉米油中,混合均匀。 配方 5 中的溶解度: 2% DMSO+30% PEG 300: 10mg/mL 配方 6 中的溶解度: 3% DMSO + 22% Castor oil + 75% Saline 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6567 mL | 13.2834 mL | 26.5668 mL | |
| 5 mM | 0.5313 mL | 2.6567 mL | 5.3134 mL | |
| 10 mM | 0.2657 mL | 1.3283 mL | 2.6567 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02569320 | Active Recruiting |
Drug: Entinostat Drug: Nivolumab |
Renal Cell Carcinoma | Roberto Pili | August 31, 2018 | Phase 2 |
| NCT02936752 | Active Recruiting |
Drug: Entinostat Biological: Pembrolizumab |
Myelodysplastic Syndrome | National Cancer Institute (NCI) |
April 3, 2017 | Phase 1 |
| NCT03501381 | Active Recruiting |
Drug: Entinostat Drug: Interleukin-2 |
Renal Cell Carcinoma | Roberto Pili | May 24, 2018 | Phase 2 |
| NCT03978624 | Active Recruiting |
Drug: Pembrolizumab Drug: Entinost |
Bladder Cancer | UNC Lineberger Comprehensive Cancer Center |
September 23, 2020 | Phase 2 |
| NCT03280563 | Active Recruiting |
Drug: Entinostat Drug: Exemestane |
Breast Neoplasms | Hoffmann-La Roche | December 26, 2017 | Phase 1 Phase 2 |
|
|---|
 |
 |