| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体内研究 (In Vivo) |
一种抗癫痫药物是醋酸艾斯利卡西平。它是一种前药,可转化为艾斯利卡西平,也称为 S-利卡西平,是一种活性奥卡西平代谢物。 ..因此,其作用方式与奥卡西平相同。理论上,奥卡西平可能会在给药后立即产生比醋酸艾斯利卡西平更高的 (S)-(+)-利卡西平峰值水平,这可以增强耐受性[1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Eslicarbazepine active metabolite has a high bioavailability and reaches peak serum concentration 1-4 hours after a given dose. Eslicarbazepine acetate absorption is not affected by food. Eslicarbazepine acetate and its metabolites are eliminated primarily via renal excretion. Eslicarbazepine active metabolite is excreted two-thirds in the unchanged form and one-third as a glucuronide conjugate. This accounts for around 90% of total metabolites excreted, with the remaining 10% being minor metabolites. Renal tubular reabsorption is expected to occur with eslicarbazepine. The apparent volume of distribution of eslicarbazepine is 61.3 L for a body weight of 70 kg based on population PK analysis. Renal clearance of eslicarbazepine was found to be approximately 20 mL/min in healthy subjects with normal renal function. Metabolism / Metabolites Eslicarbazepine acetate is rapidly and extensively metabolized to its major active metabolite, eslicarbazepine, via hydrolytic first-pass metabolism. Eslicarbazepine corresponds to about 92% of systemic exposure. Minor active metabolites (R)-licarbazepine and oxcarbazepine consist of <5% of systemic exposure. Active metabolites are then metabolized to inactive glucuronides that correspond to about 3% of systemic exposure. Eslicarbazepine had a moderate inhibitory effect on CYP2C19 and a mild activation of UGT1A1-mediated glucuronidation when studied in human hepatic microsomes. It has been shown to induce CYP3A4 enzymes in vivo. Biological Half-Life The apparent plasma half-life of eslicarbazepine is 10-20 hours in healthy subjects and 13-20 hours in epilepsy patients. Steady-state plasma concentrations are attained after 4 to 5 days of once daily dosing. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Eslicarbazepine is bound to plasma proteins at a relatively low rate of <40%, independent of concentration. In vitro studies have shown that plasma protein binding is not relevantly affected by the presence of other medications such as warfarin, diazepam, digoxin, phenytoin or tolbutamide. Similarly, the binding of these medications was not significantly affected by the presence of eslicarbazepine. |
| 参考文献 | |
| 其他信息 |
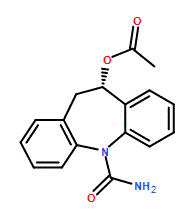
Eslicarbazepine acetate is the acetate ester, with S configuration, of licarbazepine. An anticonvulsant, it is approved for use in Europe and the United States as an adjunctive therapy for epilepsy. It has a role as an anticonvulsant and a drug allergen. It is an acetate ester, a dibenzoazepine, a carboxamide and a member of ureas. It is functionally related to a licarbazepine.
Eslicarbazepine acetate (ESL) is an anticonvulsant medication approved for use in Europe, the United States and Canada as an adjunctive therapy for partial-onset seizures that are not adequately controlled with conventional therapy. Eslicarbazepine acetate is a prodrug that is rapidly converted to eslicarbazepine, the primary active metabolite in the body. Eslicarbazepine's mechanism of action is not well understood, but it is known that it does exert anticonvulsant activity by inhibiting repeated neuronal firing and stabilizing the inactivated state of voltage-gated sodium channels, thus preventing their return to the activated state during which seizure activity can occur. Eslicarbazepine acetate is marketed as Aptiom in North America and Zebinix or Exalief in Europe. It is available in 200, 400, 600, or 800mg tablets that are taken once daily, with or without food. Eslicarbazepine acetate is associated with numerous side effects including dizziness, drowsiness, nausea, vomiting, diarrhea, headache, aphasia, lack of concentration, psychomotor retardation, speech disturbances, ataxia, depression and hyponatremia. It is recommended that patients taking eslicarbazepine acetate be monitored for suicidality. See also: Eslicarbazepine (has active moiety). Drug Indication Eslicarbazepine acetate is indicated for the treatment of partial-onset seizures in patients 4 years of age and older. FDA Label Zebinix is indicated as adjunctive therapy in adults, adolescents and children aged above 6 years, with partial-onset seizures with or without secondary generalisation. Exalief is indicated as adjunctive therapy in adults with partial-onset seizures with or without secondary generalisation. Treatment of epilepsy with partial-onset seizures Mechanism of Action Eslicarbazepine acetate is converted to the active metabolite eslicarbazepine which carries out its anticonvulsant activity. The exact mechanism of action is unknown, but it is thought to involve the inhibition of voltage-gated sodium channels. In in vitro electrophysiological studies, eslicarbazepine was shown to inhibit repeated neuronal firing by stabilizing the inactivated state of voltage-gated sodium channels and preventing their return to the activated state. In vitro studies also showed eslicarbazepine inhibiting T-type calcium channels, which likely also has a role in anticonvulsant activity. Pharmacodynamics Eslicarbazepine acetate is associated with a dose- and concentration-dependant increase in heart rate and prolongation of PR interval. |
| 分子式 |
C17H16N2O3
|
|---|---|
| 分子量 |
296.3205
|
| 精确质量 |
296.116
|
| CAS号 |
236395-14-5
|
| PubChem CID |
179344
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
427.4±55.0 °C at 760 mmHg
|
| 熔点 |
183-185ºC
|
| 闪点 |
212.3±31.5 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.655
|
| LogP |
1.7
|
| tPSA |
72.63
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
440
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(=O)O[C@H]1CC2=CC=CC=C2N(C3=CC=CC=C13)C(=O)N
|
| InChi Key |
QIALRBLEEWJACW-INIZCTEOSA-N
|
| InChi Code |
InChI=1S/C17H16N2O3/c1-11(20)22-16-10-12-6-2-4-8-14(12)19(17(18)21)15-9-5-3-7-13(15)16/h2-9,16H,10H2,1H3,(H2,18,21)/t16-/m0/s1
|
| 化学名 |
[(5S)-11-carbamoyl-5,6-dihydrobenzo[b][1]benzazepin-5-yl] acetate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~337.47 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 10 mg/mL (33.75 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 100.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 10 mg/mL (33.75 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 100.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 10 mg/mL (33.75 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3747 mL | 16.8737 mL | 33.7473 mL | |
| 5 mM | 0.6749 mL | 3.3747 mL | 6.7495 mL | |
| 10 mM | 0.3375 mL | 1.6874 mL | 3.3747 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Eslicarbazepine Acetate (BIA 2-093) as Monotherapy in Patients With Newly Diagnosed Partial-onset Seizures
CTID: NCT02484001
Phase: Phase 3 Status: Completed
Date: 2018-10-16