| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
体外活性:使用 G-1 进行的 MTT 测定表明,在 SkBr3 细胞中,100 nM G-1 诱导的增殖作用在 1 μM 雌三醇(作为 GPR30 依赖性途径的拮抗剂)存在下被消除。无细胞转录测定表明,雌三醇表现出的抗雌激素活性是由于干扰雌二醇诱导的正协同结合和受体二聚化、hER 复合物与 ERE 的结合,以及以剂量依赖性方式减少雌二醇依赖性转录。 。最近的一项研究表明,雌激素(雌酮、雌二醇和雌三醇)可抑制阿尔茨海默病相关的低阶 Aβ 寡聚体形成,其中雌三醇表现出最强的体外活性。细胞测定:对于 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 测定,细胞在塑料 96 孔板中在 200 μL 生长培养基中以初始密度培养每孔 10,000 个细胞。细胞附着后进行清洗,并在含有 2.5% 木炭剥离的 FBS 的培养基中进一步孵育并进行指定处理。每 2 天更换一次培养基(处理时)。如果适用,按照制造商的建议,在使用 Fugene6 试剂处理之前每 2 天转染 200 ng 指定质粒。孵育 6 天后,将含有 1mg/mL 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物 (MTT) 的测定混合物(每孔 10μL)添加到每个孔中,并在 37 ℃下孵育。 ◦C 在 5% CO2 气氛中保持 4 小时。黄色四唑 MTT 被代谢活跃的细胞还原,产生细胞内紫色甲臜,与 200 µL 1% 十二烷基硫酸钠的 0.01N HCL 溶液过夜孵育后释放,并通过使用酶联免疫吸附测定读取 570 nm 处的吸光度进行分光光度定量读板器。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
在 mPTEN+/- 小鼠中,雌三醇治疗导致子宫湿重与体重的相对比增加 187.54%;雌三醇还将野生型小鼠的这一比例提高至 176.88%。体内雌三醇治疗(20 mg/kg ip)可通过依赖于大鼠肠道通透性增加导致门静脉血内毒素升高导致 CD14 增加的机制,使 Kupffer 细胞对 LPS 敏感。而在注射亚致死剂量的LPS(5mg/kg)前24小时腹腔注射雌三醇的大鼠中有一半在24小时内死亡。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Estriol is readily absorbed following intravaginal application. Peak serum estriol concentrations are generally observed within 2 hours following intravaginal application and remain elevated for 6 hours. Systemic bioavailability on vaginal administration is better than after oral administration. Intravaginal application of 1 mg estriol in women with senile atrophy of the vaginal epithelium results in serum levels similar to those seen after oral administration of 10 mg estriol. Plasma estriol levels increased from <90pmol/L (26 pg/mL) about fifty fold over a few hours after intravaginal administration of Gynest Cream. Eight to ten hours after administration, 50% of women still had estriol levels above 90pmol/L (26 pg/mL). Estriol circulates with the blood, about 14% free, 8% bound to SHBG and the rest bound to albumin. More than 95% of estriol is excreted in the urine, predominantly in the form of glucuronides. Metabolism / Metabolites Primary metabolites of estriol include the 16-alpha-glucuronide, 3-glucuronide, 3-sulfate and 3-sulfate 16-alpha-glucuronide. The metabolic disposition of estrogens includes oxidative metabolism (largely hydroxylation) and conjugative metabolism by glucuronidation, sulfonation and/or O-methylation. Estradiol is converted to estrone by a 17beta-hydroxysteroid dehydrogenase; the estrone produced is further metabolized to 16alpha-hydroxyoestrone and then to estriol. Estriol is a common metabolite of estrone and estradiol-17-beta in animals and in humans. Estriol is excreted in humans as conjugated and unconjugated 2-hydroxy estriol after 2-hydroxylation. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Estriol levels can be measured to give an indication of the general health of the fetus. DHEA-S is produced by the adrenal cortex of the fetus. This is converted to estriol by the placenta. If levels of "unconjugated estriol" are abnormally low in a pregnant woman, this may indicate a problem with the development of the child. The drug interacts with a target cell receptor. When the estrogen receptor has bound its ligand it can enter the nucleus of the target cell, and regulate gene transcription which leads to formation of messenger RNA. The mRNA interacts with ribosomes to produce specific proteins that express the effect of estriol upon the target cell. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. Toxicity Data ORAL (LD50): Acute: >2000 mg/kg [Rat]. |
||
| 参考文献 |
Mol Cell Endocrinol.2010 May 14;320(1-2):162-70;Lab Invest.2006 Mar;86(3):286-96.
|
||
| 其他信息 |
Therapeutic Uses
/Estriol is indicated as/ hormone replacement therapy for treatment of atrophic vaginitis and kraurosis in post-menopausal women. /Estriol is indicated for the/ treatment of pruritus vulvae and dyspareunia associated with atrophic vaginal epithelium. Drug Warnings Gynest Cream is not indicated during pregnancy. If pregnancy occurs during use of Gynest Cream, treatment should be withdrawn immediately. Gynest Cream contains arachis oil (peanut oil) and should not be applied by patients known to be allergic to peanuts. As there is a possible relationship between allergy to peanuts and allergy to soya, patients with soya allergy should also avoid Gynest Cream. Before initiating or re-instituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contra-indications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised what changes in their breasts should be reported to their doctor or nurse. Investigations, including mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual. The risk of endometrial hyperplasia and carcinoma is increased when systemic estrogens are administered alone for prolonged periods of time. The endometrial safety of long-term or repeated use of topical vaginal estrogens is uncertain. Therefore, if repeated, treatment should be reviewed at least annually, with a special consideration given to any symptoms of endometrial hyperplasia or carcinoma. For more Drug Warnings (Complete) data for ESTRIOL (40 total), please visit the HSDB record page. Pharmacodynamics Estriol (also oestriol) is one of the three main estrogens produced by the human body. It is only produced in significant amounts during pregnancy as it is made by the placenta. In pregnant women with multiple sclerosis (MS), estriol reduces the disease's symptoms noticeably, according to researchers at UCLA's Geffen Medical School. |
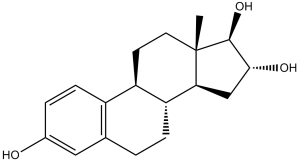
| 分子式 |
C18H24O3
|
|
|---|---|---|
| 分子量 |
288.39
|
|
| 精确质量 |
288.172
|
|
| CAS号 |
50-27-1
|
|
| 相关CAS号 |
Estriol (Standard);50-27-1;Estriol-d3;79037-36-8;Estriol-d2;53866-32-3;Estriol-d;55727-98-5;Estriol-13C3;1255639-56-5;Estriol-d3-1;2687960-79-6
|
|
| PubChem CID |
5756
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
469.0±45.0 °C at 760 mmHg
|
|
| 熔点 |
280-282 °C(lit.)
|
|
| 闪点 |
220.8±23.3 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.624
|
|
| LogP |
2.94
|
|
| tPSA |
60.69
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
411
|
|
| 定义原子立体中心数目 |
6
|
|
| SMILES |
C[C@]12CC[C@H]3[C@H]([C@@H]1C[C@H]([C@@H]2O)O)CCC4=C3C=CC(=C4)O
|
|
| InChi Key |
PROQIPRRNZUXQM-ZXXIGWHRSA-N
|
|
| InChi Code |
InChI=1S/C18H24O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-17,19-21H,2,4,6-7,9H2,1H3/t13-,14-,15+,16-,17+,18+/m1/s1
|
|
| 化学名 |
(8R,9S,13S,14S,16R,17R)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,16,17-triol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.21 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.21 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.21 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO +95%Corn oil: 10 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4675 mL | 17.3376 mL | 34.6753 mL | |
| 5 mM | 0.6935 mL | 3.4675 mL | 6.9351 mL | |
| 10 mM | 0.3468 mL | 1.7338 mL | 3.4675 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Determine Efficacy & Safety of a Low Concentration Estriol (0.005%) in Postmenopausal Vaginal Atrophy.
CTID: NCT04574999
Phase: Phase 3 Status: Completed
Date: 2020-12-11