| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ethyl (14C)-vanillin was administered to male and female Sprague-Dawley CD rats by gavage in polyethylene glycol solution at single doses of 50, 100, or 200 mg/kg bw. Ethyl vanillin was rapidly absorbed and peak plasma radioactivity occurred within 2 hr after dosing at all dose levels, falling rapidly to undetectable levels within 96 hr. Plasma radioactivity tended to be higher in female than male rats and it was postulated that this might reflect a lower metabolic capacity of female rats. Urinary excretion of radioactivity was rapid and more than 94% of the dose was excreted by this route within 24 hr. Only 1-5% of the dose was excreted in faeces. After 5 days, more than 99% of the administered dose was excreted. No radioactivity was detected in expired air, indicating that the aromatic ring was in a metabolically stable position. Metabolism / Metabolites A healthy adult male volunteer drank a 235 ml aliquot of a liquid dietary supplement containing an unknown quantity of ethyl vanillin. A concentration of 13 mg ethyl vanillic acid/g creatinine was found in a 12-hour urine sample. The compound was not present in urine collected before exposure. Ethyl vanillic acid was identified by GC/MS in the urine of a 9-year old female patient who had received liquid dietary supplementation flavored with vanilla. Other patients excreting this acid were also known to have consumed foodstuffs flavored with ethyl vanillin. Eight different urine samples containing more than 50 mg ethyl vanillic acid/g creatinine were also found to contain small amounts of vanillylmandelic acid. Unchanged ethyl vanillin was not detected in any of the urine samples. During urinary organic acid profiling in human subjects, several patients excreted high concentrations of ethyl vanillic acid (3-ethoxy-4-hydroxybenzoic acid) and traces of 3-ethoxy-4-hydroxy- mandelic acid. Ethyl (14C)-vanillin was administered to male and female Sprague Dawley CD rats at single oral doses of 50, 100, or 200 mg/kg bw. Rapid metabolism occurred and the principal metabolite at all dose levels was ethyl vanillic acid. Analysis of urine after hydrolysis with glucuronidase and/or sulfatase indicated that the major metabolites were glucuronide or sulfate conjugates of ethyl vanillic acid (56-62%), ethyl vanillyl alcohol (15-20%), and ethyl vanillin (7-12%). A minor proportion of the dose (2-8%) was excreted as the glycine conjugate of vanillic acid (ethyl vanilloyl glycine). Early reports indicated that ethyl vanillin was probably metabolized to glucuroethyl vanillin and ethyl vanillic acid, of which some was conjugated with glucuronic and sulfuric acids. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Ethyl vanillin forms fine crystalline needles and is used in fragrances for its vanilla odor. It is also used as a replacement of or to strengthen the flavor of vanilla in a variety of foods. Ethyl vanillin is a widely used food additive and spice in foods, beverages, cosmetics and drugs. HUMAN EXPOSURE AND TOXICITY: Research in humans showed that ethyl vanillin had no significant effect on the activity of five human CYP450 enzymes with concentration ranged from 8 to 128 uM. A 2% concentration of ethyl vanillin caused mild irritation on the skin of humans after 48 hours of direct contact. ANIMAL STUDIES: In animal studies, ethyl vanillin was found to be mostly non-toxic except when given at high doses for longer than 6 weeks. Rabbits were given ethyl vanillin orally in 10% aqueous glycerin 49 mg/kg bw/day for 43 days. At this dose level anemia, diarrhea and lack of weight gain were observed. Rats (20/sex/group) were fed ethyl vanillin of > 99.9% purity (nature of diet e.g., semi-synthetic/chow diet, not specified) at dose levels of 0, 500, 1000 or 2000 mg/kg bw/day for 13 weeks. Clinical biochemistry showed statistically significant higher values in the high-dose group compared to control for ALAT, ALP, cholesterol and total plasma protein. Histological examination revealed a dose-related increased incidence of hepatic peribiliary inflammatory change in both males and females of the intermediate- and high-dose groups, and minor bile duct hyperplasia affecting 1/20 intermediate- and 4/20 high-dose males. There were no changes observed in the liver parenchyma and no degenerative or inflammatory changes of the bile duct epithelium. Increased white pulp cellularity and prominence of germinal centers in the spleen, and increased prominence of germinal centers and lymphoid proliferation in cervical lymph nodes were seen in the intermediate- and high-dose groups. Groups of 12 male and 12 female rats were fed diets containing 0, 0.5, 1 and 2% ethyl vanillin for 2 years or 2% and 5% for one year without any adverse effects on growth, organ weights of major organs, hematology and histology of major tissues. In genotoxicity studies, ethyl vanillin did not induce genetic changes in vitro but was reported to enhance the ability of mitomycin C to cause sister chromatid exchanges. Ethyl vanillin has shown to have anti-angiogenic, anti-inflammatory and anti-nociceptive properties that are based on its suppressive effect on the production of nitric oxide possibly via decreasing the reactive oxygen species level. The in vivo results revealed that drug interaction between vanillin/ethyl vanillin and drugs metabolized by CYP2E1 or CYP1A2 might be possible, and also suggested that the application of the above additives in foods and drugs should not be unlimited so as to avoid the adverse interaction. The thermal tolerance Cronobacter sakazakii was examined in sterile powdered infant formula (PIF) rehydrated at 58 °C in water or apple juice supplemented with vanillin, ethyl vanillin, or vanillic acid. All three compounds decreased thermal tolerance during-rehydration. Supplementation of PIF with vanillin, ethyl vanillin, or vanillic acid could enhance the safety of PIF or other dehydrated foods contaminated with C. sakazakii. Non-Human Toxicity Values LD50 Dog iv 760 mg/kg LD50 Rat sc 1800 mg/kg LD50 Rabbit oral 3000 mg/kg LD50 Rat oral >2000 mg/kg For more Non-Human Toxicity Values (Complete) data for ETHYL VANILLIN (7 total), please visit the HSDB record page. |
| 其他信息 |
Ethyl vanillin appears as colorless crystals. More intense vanilla odor and taste than vanillin. (NTP, 1992)
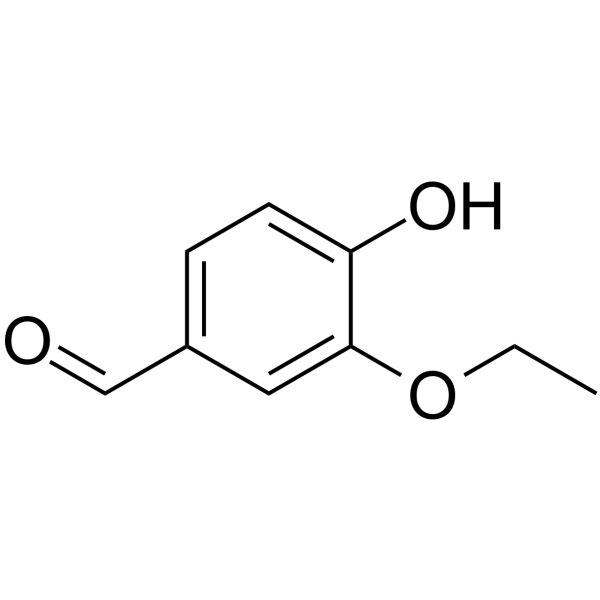
Ethyl vanillin is a member of the class of benzaldehydes that is vanillin in which the methoxy group is replaced by an ethoxy group. It has a role as an antioxidant and a flavouring agent. It is a member of benzaldehydes, a member of phenols and an aromatic ether. It is functionally related to a vanillin. Ethyl vanillin has been reported in Microtropis japonica and Cornus officinalis with data available. Ethyl vanillin is a metabolite found in or produced by Saccharomyces cerevisiae. |
| 分子式 |
C9H10O3
|
|---|---|
| 分子量 |
166.1739
|
| 精确质量 |
166.062
|
| CAS号 |
121-32-4
|
| 相关CAS号 |
Ethylvanillin-d5;1335401-74-5
|
| PubChem CID |
8467
|
| 外观&性状 |
Fine, crystalline needles
White or slightly yellowish crystals Colorless flakes |
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
295.1±20.0 °C at 760 mmHg
|
| 熔点 |
76 °C
|
| 闪点 |
119.0±15.3 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.574
|
| LogP |
1.72
|
| tPSA |
46.53
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
147
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])C([H])([H])[H])C1=C(C([H])=C([H])C(C([H])=O)=C1[H])O[H]
|
| InChi Key |
CBOQJANXLMLOSS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H10O3/c1-2-12-9-5-7(6-10)3-4-8(9)11/h3-6,11H,2H2,1H3
|
| 化学名 |
3-ethoxy-4-hydroxybenzaldehyde
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol :≥ 100 mg/mL (~601.79 mM)
DMSO : ≥ 100 mg/mL (~601.79 mM) H2O : ~5 mg/mL (~30.09 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (15.04 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (15.04 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (15.04 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.0179 mL | 30.0897 mL | 60.1793 mL | |
| 5 mM | 1.2036 mL | 6.0179 mL | 12.0359 mL | |
| 10 mM | 0.6018 mL | 3.0090 mL | 6.0179 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。