| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 靶点 |
Cyclooxygenase-1 (COX-1) (IC50: 1.8 ± 0.2 μM for Etodolac (AY-24236)) [2]
- Cyclooxygenase-2 (COX-2) (IC50: 0.25 ± 0.03 μM for Etodolac (AY-24236), selectivity ratio (COX-1/COX-2) = 7.2) [2,3] |
|---|---|
| 体外研究 (In Vitro) |
上市后研究表明,依托度酸的环氧合酶抑制作用对 COX-2 有一定的选择性,就像塞来昔布和其他“COX-2 抑制剂”一样。与罗非考昔相比,依托度酸和塞来昔布都被归类为对 COX-2 具有“优先选择性”,并且可以完全抑制 COX-1。在肝癌细胞中,依托度酸的 r 对映体(对 COX 无活性)可抑制 β-连环蛋白的表达。
1. 环氧合酶(COX)抑制活性:依托度酸(Etodolac, AY-24236) 对COX-1和COX-2表现出浓度依赖性抑制。其对COX-2的IC50(0.25±0.03 μM)显著低于对COX-1的IC50(1.8±0.2 μM),COX-1/COX-2选择性比值为7.2。相比之下,吲哚美辛(非选择性COX抑制剂)的COX-1/COX-2选择性比值为0.8,证实依托度酸对COX-2具有偏好性[2] 2. 抑制胃癌前病变细胞增殖:从广泛性肠化型胃炎患者中分离原代胃黏膜细胞,用依托度酸(0.1-10 μM)处理48小时。MTT实验显示,1 μM 依托度酸使细胞活力降低18.3±2.5%,10 μM时抑制率增至35.6±3.8%。Western blot结果显示,与对照组相比,10 μM 依托度酸使COX-2和前列腺素E2(PGE2)合酶的表达分别下调42.1±4.3%和38.5±3.9%[3] 3. 抑制炎症细胞因子生成:用脂多糖(LPS)刺激RAW264.7巨噬细胞,再用依托度酸(1-20 μM)处理24小时。酶联免疫吸附实验(ELISA)显示,与仅LPS处理组相比,10 μM 依托度酸使肿瘤坏死因子-α(TNF-α)分泌减少52.3±5.1%,白细胞介素-6(IL-6)分泌减少47.8±4.8%;浓度≤1 μM时对细胞因子生成无显著影响[4] |
| 体内研究 (In Vivo) |
在机械性异常性疼痛小鼠模型中,依托度酸通过不依赖于 COX 的途径减轻紫杉醇诱导的周围神经病变。依托度酸和其他非甾体抗炎药以剂量依赖性方式抑制佐剂关节炎大鼠的爪肿胀并引起胃粘膜损伤。在这些非甾体抗炎药中,依托度酸在关节炎大鼠中显示出最高的 UD(50) 值和安全指数。依托度酸还显示出最高的 UD(50) 值和安全指数,除非通过乙酸诱导的正常大鼠扭体来评估其效果。依托度酸剂量依赖性地抑制胃癌的发展,在 30 mg/kg/天的剂量下没有检测到癌症。依托度酸不影响炎症细胞浸润或氧化性 DNA 损伤的程度,但它显着抑制粘膜细胞增殖,并以剂量依赖性方式抑制幽门螺杆菌 (Hp) 感染的蒙古沙鼠 (MG) 胃中肠化生的发展。依托度酸减轻CCI大鼠热诱发的痛觉过敏,CCI侧TRAP阳性多核破骨细胞数量的增加被消除,但不抑制骨矿物质含量(BMC)和骨矿物质密度(BMD)的降低。 )在 CCI 端。
1. 减轻紫杉醇诱导的周围神经病变(小鼠模型):雄性ICR小鼠(25-30 g)每隔1天腹腔注射紫杉醇(2 mg/kg),共5次(总剂量10 mg/kg),诱导机械性痛觉过敏。从第1天到第14天,每天口服给予依托度酸(Etodolac, AY-24236) 10 mg/kg、30 mg/kg或100 mg/kg。第14天,30 mg/kg和100 mg/kg 依托度酸组的机械撤退阈值(用von Frey纤维测定)分别为8.5±0.7 g和10.2±0.9 g,显著高于仅紫杉醇组(4.1±0.5 g);10 mg/kg组无显著效果(5.2±0.6 g)。此外,30 mg/kg 依托度酸使腰段脊髓中COX-2和PGE2的表达分别降低39.2±4.1%和45.6±4.7%[2] 2. 预防胃癌前病变的癌变(大鼠模型):雄性Wistar大鼠(180-220 g)饮用含N-甲基-N'-硝基-N-亚硝基胍(MNNG,100 μg/mL)的水12周,诱导广泛性肠化型胃炎(癌前病变)。从第13周到第24周,大鼠每天口服依托度酸 10 mg/kg或30 mg/kg。第24周,30 mg/kg 依托度酸组的胃癌发生率(12.5%)显著低于仅MNNG组(45.8%);10 mg/kg组的癌症发生率为28.3%(与MNNG组无统计学差异)。30 mg/kg组胃黏膜PGE2水平较仅MNNG组降低51.2±5.3%[3] 3. 延长心脏移植移植物存活时间(小鼠模型):向Balb/c小鼠(受体,雌性,20-25 g)移植C57BL/6小鼠(供体,雄性,20-25 g)的异位心脏。移植前1天开始,每天口服给予依托度酸 30 mg/kg或60 mg/kg,持续21天。60 mg/kg 依托度酸组的移植物中位存活时间为18.5±1.2天,显著长于溶剂组(8.2±0.8天);30 mg/kg组中位存活时间为12.3±1.0天(与溶剂组相比有统计学意义)。流式细胞术显示,60 mg/kg 依托度酸使浸润移植物的CD4+和CD8+ T细胞数量分别减少42.3±4.5%和38.7±4.2%[4] |
| 酶活实验 |
1. COX-1活性测定实验:从绵羊精囊腺中提取COX-1。反应体系(200 μL)包含50 mM Tris-HCl缓冲液(pH 8.0)、2 μM血红素、100 μM花生四烯酸(底物)以及系列稀释的依托度酸(Etodolac, AY-24236)(0.01-10 μM)。混合物在37°C孵育15分钟,加入20 μL 1 M HCl终止反应。采用竞争性酶免疫测定(EIA)试剂盒检测PGE2(COX-1的主要产物)浓度,抑制率按(1 - 样品PGE2浓度/对照PGE2浓度)×100%计算,通过非线性回归分析确定IC50[2]
2. COX-2活性测定实验:使用Sf9昆虫细胞表达的重组人COX-2。反应条件与COX-1测定一致,不同之处在于反应缓冲液中加入10 μM双氯芬酸(作为COX-1抑制剂,以排除COX-1污染)。孵育和终止反应后,通过EIA检测PGE2水平,采用与COX-1相同的方法计算依托度酸对COX-2的IC50[2,3] |
| 细胞实验 |
1. 原代胃黏膜细胞活力测定(MTT法):将广泛性肠化型胃炎患者的胃黏膜组织切碎,用0.1%胶原酶在37°C消化2小时。单细胞经70 μm细胞筛过滤后,重悬于含10%胎牛血清的RPMI 1640培养基中。以5×10³个细胞/孔接种于96孔板,过夜培养。加入依托度酸(Etodolac, AY-24236)(0.1-10 μM),继续培养48小时。每孔加入20 μL MTT溶液(5 mg/mL),再孵育4小时。去除上清液,加入150 μL DMSO溶解甲臜结晶,检测570 nm处吸光度。细胞活力按(样品吸光度/对照吸光度)×100%计算[3]
2. 胃细胞中COX-2和PGE2合酶的Western blot实验:将原代胃黏膜细胞以2×10⁵个细胞/孔接种于6孔板,用10 μM 依托度酸处理48小时。用含蛋白酶抑制剂的RIPA裂解液裂解细胞,BCA法测定蛋白浓度。取等量蛋白(40 μg)进行10% SDS-PAGE电泳,转移至PVDF膜。膜用5%脱脂牛奶封闭1小时,随后与抗COX-2、抗PGE2合酶和抗GAPDH(内参)一抗在4°C孵育过夜。TBST洗涤后,膜与辣根过氧化物酶偶联的二抗孵育1小时。用ECL试剂显影条带,ImageJ软件定量条带强度[3] 3. 巨噬细胞炎症细胞因子的ELISA实验:将RAW264.7细胞以1×10⁵个细胞/孔接种于24孔板,用1 μg/mL LPS刺激2小时。加入依托度酸(1-20 μM),继续培养22小时。收集培养上清液,使用商品化ELISA试剂盒按说明书检测TNF-α和IL-6浓度,结果以仅LPS处理组为对照进行标准化[4] |
| 动物实验 |
30 mg/kg
Mice 1. Paclitaxel-induced peripheral neuropathy model (ICR mice): - Animals: Male ICR mice (n=8/group), 25-30 g. - Model induction: Intraperitoneal injection of paclitaxel (2 mg/kg) every other day for 5 doses (days 1, 3, 5, 7, 9), total dose 10 mg/kg. - Drug administration: Etodolac (AY-24236) was dissolved in 0.5% carboxymethyl cellulose (CMC-Na) and orally administered once daily at 10 mg/kg, 30 mg/kg, or 100 mg/kg from day 1 to day 14. The vehicle group received 0.5% CMC-Na alone. - Evaluation: Mechanical withdrawal threshold was measured using von Frey filaments on days 1, 7, and 14. On day 14, mice were sacrificed, and lumbar spinal cord tissues were collected for Western blot analysis of COX-2 and PGE2 [2] 2. Gastric precancerous lesion model (Wistar rats): - Animals: Male Wistar rats (n=10/group), 180-220 g. - Model induction: Drinking water containing MNNG (100 μg/mL) for 12 weeks to induce extensive metaplastic gastritis. - Drug administration: From week 13 to week 24, etodolac was dissolved in 0.5% CMC-Na and orally administered once daily at 10 mg/kg or 30 mg/kg. The MNNG-only group received 0.5% CMC-Na. - Evaluation: At week 24, rats were sacrificed. Gastric tissues were examined for cancer incidence by histopathology, and gastric mucosal PGE2 levels were measured by EIA [3] 3. Cardiac allograft model (Balb/c and C57BL/6 mice): - Animals: Recipients: Balb/c mice (female, n=6/group), 20-25 g; Donors: C57BL/6 mice (male), 20-25 g. - Model induction: Heterotopic cardiac transplantation (abdominal aorta and inferior vena cava anastomosis) under isoflurane anesthesia. - Drug administration: Etodolac was dissolved in 0.5% CMC-Na and orally administered once daily at 30 mg/kg or 60 mg/kg, starting 1 day before transplantation and continuing for 21 days. The vehicle group received 0.5% CMC-Na. - Evaluation: Allograft survival was monitored by daily abdominal palpation (cessation of heartbeat = rejection). At rejection, grafts were collected for flow cytometry (CD4+ and CD8+ T cell infiltration) [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Based on mass balance studies, the systemic bioavailability of etodolac from either the tablet or capsule formulation is at least 80%. It is not known whether etodolac is excreted in human milk; however, based on its physical-chemical properties, excretion into breast milk is expected. Etodolac is extensively metabolized in the liver. The hydroxylated-etodolac metabolites undergo further glucuronidation followed by renal excretion and partial elimination in the feces (16% of dose). Approximately 1% of a etodolac dose is excreted unchanged in the urine with 72% of the dose excreted into urine as parent drug plus metabolite. 390 mL/kg Oral cl=49.1 mL/h/kg [Normal healthy adults] Oral cl=49.4 mL/h/kg [Healthy males (18-65 years)] Oral cl=35.7 mL/h/kg [Healthy females (27-65 years)] Oral cl=45.7 mL/h/kg [Eldery (>65 years)] Oral cl=58.3 mL/h/kg [Renal impairement (46-73 years)] Oral cl=42.0 mL/h/kg [Hepatic impairement (34-60 years)] Metabolism / Metabolites Etodolac is extensively metabolized in the liver. Renal elimination of etodolac and its metabolites is the primary route of excretion (72%). Metabolites found in urine (with percents of the administered dose) are: unchanged etodolac (1%), etodolac glucuronide (13%), hydroxylated metabolites (6-, 7-, and 8-OH; 5%), hydroxylated metabolite glucuronides (20%), and unidentified metabolites (33%). Fecal excretion accounts for 16% of its elimination. Etodolac has known human metabolites that include (2S,3S,4S,5R)-6-[2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid. Biological Half-Life Terminal t1/2, 7.3 ± 4.0 hours. Distribution t1/2, 0.71 ± 0.50 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Prospective studies show that 1% to 2% of patients taking etodolac experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in Likelihood score: C (probable rare cause clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of etodolac during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding > 99% bound, primarily to albumin 1. In vivo general toxicity: In the 24-week rat gastric precancerous lesion study, Etodolac (AY-24236) at 10 mg/kg and 30 mg/kg/day had no significant effect on rat body weight (final weight: 385 ± 25 g and 378 ± 22 g, respectively, vs. 392 ± 28 g in MNNG-only group). No gross pathological changes were observed in the liver, kidney, or stomach after necropsy [3] 2. In vivo organ toxicity: In the 21-day mouse cardiac allograft study, serum levels of alanine transaminase (ALT) and creatinine in the 60 mg/kg etodolac group (ALT: 45 ± 8 U/L; creatinine: 0.52 ± 0.06 mg/dL) were similar to those in the vehicle group (ALT: 42 ± 7 U/L; creatinine: 0.50 ± 0.05 mg/dL), indicating no significant hepatotoxicity or nephrotoxicity [4] |
| 参考文献 |
|
| 其他信息 |
Etodolac can cause developmental toxicity and female reproductive toxicity according to state or federal government labeling requirements.
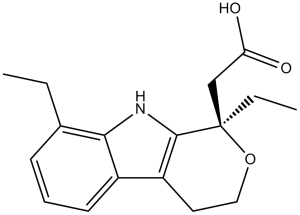
Etodolac is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl moiety. A preferential inhibitor of cyclo-oxygenase 2 and non-steroidal anti-inflammatory, it is used for the treatment of rheumatoid arthritis and osteoarthritis, and for the alleviation of postoperative pain. Administered as the racemate, only the (S)-enantiomer is active. It has a role as a non-steroidal anti-inflammatory drug, a cyclooxygenase 2 inhibitor, a non-narcotic analgesic and an antipyretic. It is a monocarboxylic acid and an organic heterotricyclic compound. Etodolac is a non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic properties. Its therapeutic effects are due to its ability to inhibit prostaglandin synthesis. It is indicated for relief of signs and symptoms of rheumatoid arthritis and osteoarthritis. Etodolac is a Nonsteroidal Anti-inflammatory Drug. The mechanism of action of etodolac is as a Cyclooxygenase Inhibitor. Etodolac is a nonsteroidal antiinflammatory drug (NSAID) that is available by prescription only and is used long term for therapy of chronic arthritis and short term for acute pain. Etodolac has been linked to rare instances of clinically apparent drug induced liver disease. Etodolac is a pyranocarboxylic acid and non-steroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activities. Etodolac inhibits the activity of cyclooxygenase I and II, thereby preventing the formation of prostaglandin which is involved in the induction of pain, fever, and inflammation. It also inhibits platelet aggregation by blocking platelet cyclooxygenase and the subsequent formation of thromboxane A2. A non-steroidal anti-inflammatory agent and cyclooxygenase-2 (COX-2) inhibitor with potent analgesic and anti-arthritic properties. It has been shown to be effective in the treatment of OSTEOARTHRITIS; RHEUMATOID ARTHRITIS; ANKYLOSING SPONDYLITIS; and in the alleviation of postoperative pain (PAIN, POSTOPERATIVE). Drug Indication For acute and long-term management of signs and symptoms of osteoarthritis and rheumatoid arthritis, as well as for the management of pain. FDA Label Mechanism of Action Similar to other NSAIDs, the anti-inflammatory effects of etodolac result from inhibition of the enzyme cycooxygenase (COX). This decreases the synthesis of peripheral prostaglandins involved in mediating inflammation. Etodolac binds to the upper portion of the COX enzyme active site and prevents its substrate, arachidonic acid, from entering the active site. Etodolac was previously thought to be a non-selective COX inhibitor, but it is now known to be 5 – 50 times more selective for COX-2 than COX-1. Antipyresis may occur by central action on the hypothalamus, resulting in peripheral dilation, increased cutaneous blood flow, and subsequent heat loss. Pharmacodynamics Etodolac is an anti-inflammatory agent with analgesic and antipyretic properties. It is used to treat osteoarthritis, rheumatoid arthritis and control acute pain. The therapeutic effects of etodolac are achieved via inhibition of the synthesis of prostaglandins involved in fever, pain, swelling and inflammation. Etodolac is administered as a racemate. As with other NSAIDs, the S-form has been shown to be active while the R-form is inactive. Both enantiomers are stable and there is no evidence of R- to S- conversion _in vivo_. 1. Etodolac (AY-24236) is a non-steroidal anti-inflammatory drug (NSAID) with selective inhibition of COX-2, which is overexpressed in inflammatory tissues and cancerous/precancerous lesions. Its therapeutic effects are mainly mediated by reducing PGE2 synthesis via COX-2 inhibition [2,3,4] 2. The attenuation of paclitaxel-induced peripheral neuropathy by etodolac may be related to the suppression of COX-2/PGE2 signaling in the spinal cord, which reduces central sensitization to pain [2] 3. The preventive effect of etodolac on gastric cancer development suggests that selective COX-2 inhibitors may be a potential chemopreventive strategy for patients with high-risk gastric precancerous lesions (e.g., extensive metaplastic gastritis) [3] 4. The prolongation of cardiac allograft survival by etodolac is associated with reduced T cell infiltration, indicating that COX-2 inhibition may modulate adaptive immune responses involved in allograft rejection [4] |
| 分子式 |
C17H21NO3
|
|
|---|---|---|
| 分子量 |
287.35
|
|
| 精确质量 |
287.152
|
|
| CAS号 |
41340-25-4
|
|
| 相关CAS号 |
(rac)-Etodolac-d3;1276197-46-6
|
|
| PubChem CID |
3308
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
507.9±45.0 °C at 760 mmHg
|
|
| 熔点 |
145-1480C
|
|
| 闪点 |
261.0±28.7 °C
|
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
|
| 折射率 |
1.597
|
|
| LogP |
3.59
|
|
| tPSA |
62.32
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
400
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
NNYBQONXHNTVIJ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H21NO3/c1-3-11-6-5-7-12-13-8-9-21-17(4-2,10-14(19)20)16(13)18-15(11)12/h5-7,18H,3-4,8-10H2,1-2H3,(H,19,20)
|
|
| 化学名 |
(RS)-2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.70 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.70 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.70 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4801 mL | 17.4004 mL | 34.8008 mL | |
| 5 mM | 0.6960 mL | 3.4801 mL | 6.9602 mL | |
| 10 mM | 0.3480 mL | 1.7400 mL | 3.4801 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05679453 | Completed | Drug: Lornoxicam 8 Mg Oral Tablet | Pain, Acute
Edema Trismus |
Kutahya Health Sciences University | July 20, 2022 | Phase 4 |
| NCT02881619 | Completed | Drug: Etodolac Drug: Placebo |
Medicaments Substances in Therapeutic Use | Flavia Pardo Salata Nahsan | November 2014 | Phase 4 |
| NCT01831687 | Completed | Drug: Etodolac Extended Release Tablets USP 600mg Drug: Etodolac Extended Release Tablets 600mg |
Fasting | IPCA Laboratories Ltd. | December 2012 | Phase 1 |
| NCT01827865 | Completed | Drug: Etodolac Extended Release Tablets USP 600mg Drug: Etodolac Extended Release Tablets 600mg |
Fasting | IPCA Laboratories Ltd. | November 2012 | Phase 1 |
| NCT01857817 | Terminated | Drug: VT-122 Drug: Placebo |
Prostatic Neoplasms | Vicus Therapeutics | June 2013 | Phase 2 |