| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Trypanosoma
|
|---|---|
| 体外研究 (In Vitro) |
非昔硝唑 (HOE 239) 的两种主要代谢物是亚砜和砜。它们的 IC50 范围为 0.7-3.3 μM (0.2-0.9 μg/ml),在体外对每种测试的寄生虫菌株均具有杀锥虫活性 [1]。
|
| 体内研究 (In Vivo) |
非昔硝唑(HOE 239;连续四天;腹腔注射 20–50 mg/kg/天或口服 25–100 mg/kg/天)具有抗锥虫特性[1]。
|
| 动物实验 |
Adult female NMRI mice weighing between 20 and 25 g T. b. rhodesiense
20, 50 mg/kg (IP) or 25, 50, 100 mg/kg (PO) IP or PO; daily; four consecutive days |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Fexinidazole is well absorbed, although the rate and extent of absorption are less than dose-proportional; after a 14-day administration schedule, the mean Cmax and AUClast increased by 1.17 and 1.34, or by 1.5 and 1.61, when the dose was either doubled or tripled. Following absorption, fexinidazole is rapidly converted to its M1 metabolite, which undergoes a slower transformation to M2 over time. This is reflected in the Tmax of fexinidazole, M1, and M2 as 4 (0-9), 4 (0-6), and 6 (0-24) hours, respectively. In healthy adults given an 1800 mg loading dose followed by 1200 mg daily over 14 days, the mean Cmax for fexinidazole was 1.6 ± 0.4 μg/mL on day 1, 0.8 ± 0.3 μg/mL on day 2, and 0.5 ± 0.2 μg/mL on day 3. The relevant values for M1 were 8.1 ± 2.2, 8.0 ± 2.3, and 5.9 ± 2.1, while for M2 they were 7.5 ± 3.3, 19.6 ± 5.4, and 12.5 ± 3.5 μg/mL. Similarly, the AUC for fexinidazole was 14.3 ± 2.6, 11.6 ± 2.2, and 7.0 ± 2.5, for M1 was 102.3 ± 28.5, 127.9 ± 49.2, and 84.2 ± 36.3, and for M2 was 110.1 ± 41.1, 391.5 ± 126.7, and 252.4 ± 73.6 μg\*h/mL. Concomitant food intake increases the Cmax and AUC of fexinidazole, M1, and M2 by 2-5 fold without significantly changing the metabolite ratios. There are no clear effects of age, renal, or hepatic impairment on absorption or plasma parameters of fexinidazole or its metabolites; further studies may be required to confirm/refute these observations. Elimination is almost entirely extra-renal; roughly 0.75-3.15% of a fexinidazole dose was recovered in urine over 168 h, primarily as M1 and M2 metabolites. Fexinidazole has an apparent volume of distribution of 3222 ± 1199 L. Fexinidazole has a mean apparent day 4 clearance of 161 ± 37 L/h. Metabolism / Metabolites Fexinidazole is metabolized by a variety of enzymes including the CYP450 enzymes CYP1A2, 2B6, 2C19, 2D6, 3A4, and 3A5 as well as flavin mono-oxygenase-3 (FMO-3). Fexinidazole is first transformed to the sulfoxide M1 and then the sulfone M2, which does not appear to undergo further metabolism. Biological Half-Life Fexinidazole, M1, and M2 have mean day 10 half-lives of 15 ± 6, 16 ± 6, and 23 ± 4 hours, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Fexinidazole therapy was not associated with elevations in aminotransferase or bilirubin levels or with clinically apparent liver injury during the ten day regimens used to treat African trypanosomiasis. However, evaluation of longer courses of therapy and use of higher doses in Chagas disease caused by Trypanosoma cruzi demonstrated several instances of ALT or AST elevations above 3 times the upper limit of normal (ULN), which persisted for as long as 3 months after stopping fexinidazole. The enzyme elevations were usually hepatocellular and arose after 2 weeks of therapy. The liver injury was asymptomatic and not associated with jaundice or with rash, fever or other signs of hypersensitivity. Nevertheless, the hepatic injury as well as delayed neutropenia led to discontinuation of the clinical trials of fexinidazole in high doses in Chagas disease. Since approval of fexinidazole for African trypanosomiasis, there have been no individual reports of liver injury associated with its use. Likelihood score: E* (unlikely but suspected cause of clinically apparent liver injury when given in the recommended regimens for African trypanosomiasis). Protein Binding Fexinidazole, M1, and M2 are approximately 98, 41, and 57 percent bound to plasma proteins, respectively. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Fexinidazole is a 2-substituted 5-nitroimidazole that is likely activated by parasitic nitroreductases to highly reactive species, leading to DNA and protein damage and eventual parasite death. The dosing schedule is designed to ensure a high enough concentration of fexinidazole and its reactive metabolites for at least 48 hours, which from _in vitro_ studies was shown to be the minimum exposure time that was effectively trypanocidal. Although fexinidazole is effective in late-stage _T. brucei gambiense_ HAT, it is less effective than NECT therapy in patients with severe (cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/μL at baseline) disease. It should only be used in these patients if there are no other available treatment options. Fexinidazole has been shown to prolong the QT interval in a dose-dependent manner and was also associated with a higher incidence of insomnia, headache, tremors, psychiatric disorders, and suicidal ideation in clinical trials; patients with pre-existing conditions or concomitant medications that could aggravate any of these effects should be treated with caution. In addition, fexinidazole has been associated with neutropenia and elevations in liver transaminases, which should be monitored. Nitroimidazoles like fexinidazole have been associated with a disulfiram-like reaction when used concomitantly with alcohol and psychotic reactions when taken with [disulfiram] itself; patients should avoid alcohol and [disulfiram] when taking fexinidazole. |
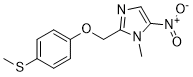
| 分子式 |
C12H13N3O3S
|
|---|---|
| 分子量 |
279.314
|
| 精确质量 |
279.067
|
| 元素分析 |
C, 51.60; H, 4.69; N, 15.04; O, 17.18; S, 11.48
|
| CAS号 |
59729-37-2
|
| PubChem CID |
68792
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
511.3±40.0 °C at 760 mmHg
|
| 闪点 |
263.0±27.3 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.629
|
| LogP |
2.28
|
| tPSA |
98.17
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
305
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])C1=NC([H])=C([N+](=O)[O-])N1C([H])([H])[H]
|
| InChi Key |
MIWWSGDADVMLTG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C12H13N3O3S/c1-14-11(13-7-12(14)15(16)17)8-18-9-3-5-10(19-2)6-4-9/h3-7H,8H2,1-2H3
|
| 化学名 |
1-methyl-2-[(4-methylsulfanylphenoxy)methyl]-5-nitroimidazole
|
| 别名 |
Hoe239; Hoe 239; Fexinidazole; Hoe-239
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50~56 mg/mL (179.0~200.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.95 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (8.95 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5803 mL | 17.9013 mL | 35.8025 mL | |
| 5 mM | 0.7161 mL | 3.5803 mL | 7.1605 mL | |
| 10 mM | 0.3580 mL | 1.7901 mL | 3.5803 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05607173 | Completed | Drug: Fexinidazole (HOE239) |
Hepatic Function Abnormal | Sanofi | January 18, 2023 | Phase 1 |
| NCT02571062 | Completed | Drug: Fexinidazole | Trypanosomiasis, African | Drugs for Neglected Diseases | March 2015 | Phase 1 |
| NCT03025789 | Completed | Drug: Fexinidazole | Trypanosomiasis, African Sleeping Sickness |
Drugs for Neglected Diseases | November 10, 2016 | Phase 3 |
| NCT02169557 | Completed | Drug: Fexinidazole | Human African Trypanosomiasis (HAT) |
Drugs for Neglected Diseases | April 30, 2014 | Phase 2 Phase 3 |
| NCT01340157 | Completed | Drug: Fexinidazole | PK in Healthy Volunteers | Drugs for Neglected Diseases | February 2011 | Phase 1 |