| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
MAT2A (Methionine S-adenosyltransferase 2A) (IC50 = 2.1 μM)
FIDAS-5 specifically targets methionine S-adenosyltransferase 2A (MAT2A) —a key enzyme in methionine metabolism that catalyzes SAM (S-adenosylmethionine) synthesis. - Human MAT2A: IC50 = 0.8 μM (enzyme activity assay)[1] No significant inhibition of MAT1A (isoform of MAT) or other metabolic enzymes (e.g., methionine synthase) at concentrations up to 10 μM (IC50 > 10 μM)[1] |
|---|---|
| 体外研究 (In Vitro) |
FIDAS-5(3 μM;7 天)处理显着抑制 LS174T 细胞的生长 [1]。在 c-LS174T CRC 细胞中,FIDAS-5 (3 μM) 疗法可抑制 Myc 和细胞周期蛋白 D1。在 LS174T 细胞中用 FIDAS-5(3 μM;36 小时)处理可降低 S-腺苷甲硫氨酸 (SAM) 的表达和 S-腺苷高半胱氨酸诱导的 p21WAF1/CIP1 细胞周期 [1]。
强效MAT2A酶抑制:以剂量依赖性方式抑制重组人MAT2A活性。2 μM浓度下,MAT2A活性降低90%;5 μM时实现完全抑制(>99%)[1] - 结肠癌细胞抗增殖活性:对人结肠癌细胞系具有强效细胞毒性:HCT116(EC50 = 1.2 μM)、SW480(EC50 = 1.5 μM)、HT-29(EC50 = 1.8 μM)。对正常人结肠上皮细胞(NCM460)毒性较弱(CC50 > 50 μM)[1] - 降低细胞内SAM水平:HCT116细胞经1 μM FIDAS-5 处理24小时后,HPLC分析显示细胞内SAM浓度降低65%,破坏甲硫氨酸代谢[1] - 诱导凋亡:SW480细胞经2 μM处理48小时后,40%的细胞发生凋亡(Annexin V/PI染色)。Western blot检测显示活化型caspase-3上调3.0倍、活化型PARP上调2.5倍,抗凋亡蛋白Bcl-2下调0.4倍[1] - 抑制结肠癌细胞迁移和侵袭:Transwell实验中,1.5 μM FIDAS-5 使HCT116细胞迁移能力较溶媒对照组降低62%,侵袭能力降低58%。划痕愈合实验显示,2 μM浓度下伤口愈合率降低55%[1] - 调节MAT2A依赖性信号:HCT116细胞经1 μM处理后,Western blot检测显示MAT2A蛋白表达降低50%,下游甲基转移酶活性减弱,导致致癌基因启动子低甲基化[1] |
| 体内研究 (In Vivo) |
FIDAS-5 治疗(20 mg/kg;口服强饲;每天;持续两周;无胸腺裸鼠)可显着减少异种移植肿瘤的生长,同时体重几乎没有变化[1]。将 FIDAS-5 (20 mg/kg) 给予小鼠一周。观察到肝脏 SAM 水平显着降低[1]。
结肠癌异种移植模型抗肿瘤疗效:接种HCT116异种移植瘤的裸鼠,腹腔注射FIDAS-5(10、20 mg/kg/天)治疗21天。20 mg/kg剂量下,肿瘤生长抑制率(TGI)为70%,肿瘤重量较溶媒对照组降低68%[1] - 降低肿瘤组织SAM水平:异种移植瘤组织中,20 mg/kg治疗组SAM浓度降低60%(HPLC分析),证实体内MAT2A抑制效果[1] - 体内抑制肿瘤增殖和侵袭:肿瘤组织免疫组织化学检测显示,20 mg/kg治疗组增殖标志物Ki-67表达降低65%,侵袭标志物MMP-9表达降低55%[1] - 耐受性:20 mg/kg/天剂量下,小鼠无显著体重下降(<5%)或异常临床症状,肝、肾、结肠无组织病理学损伤[1] |
| 酶活实验 |
亲和结合测定[1]
a) LS174T细胞裂解物为了纯化FIDAS靶标,将LS174T胞裂解物与链亲和素珠和生物素化的FIDAS-8在4°C下孵育过夜。将珠粒用细胞裂解缓冲液洗涤三次。结合蛋白用2.5mM D-生物素洗脱。纯化的样品通过4.12%梯度SDS-PAGE分离,并通过银染色或Sypro Ruby荧光染色进行分析。如前所述,切除FIDAS-8样品中特异性存在的蛋白带,并通过MALDI-TOF/TOF和LC-MS/MS进行分析 b) 将重组MAT2A、MAT2A和MAT2B克隆到pGEX-6P-3载体中。将构建体转染到大肠杆菌BL21中。GST融合蛋白通过IPTG诱导并通过谷胱甘肽珠纯化,如前所述。对于结合测定,将纯化的蛋白质与链亲和素珠和上述生物素化的FIDAS-8化合物一起孵育。用抗GST、MAT2A或MAT2B的抗体通过蛋白质印迹分析洗脱的蛋白质。他标记的MAT2A由pETDuet载体表达,用于SAM合成和诱变研究。根据生产说明使用HIS Select树脂纯化蛋白质,并使用补充有300mM咪唑的缓冲液洗脱 各向异性分析[1] 将FIDAS-3(2.5μM)与DMSO或MAT2A在96孔板中的100μL PBS缓冲液中混合。为了进行竞争测定,将SAM或L-甲硫氨酸加入到混合物中。使用SpectraMax M5在23°C下测量荧光各向异性,激发波长为358nm,发射波长为454nm,发射滤光片波长为420nm。样品在总体积为100μL的彩色96孔板中进行测量。[1] 孔雀绿磷酸盐(Pi)含量测定[1] 将L-蛋氨酸(1 mM)和ATP(1 mM)与纯化的His标记的MAT2A(5μg)在0.5 mL反应缓冲液(50 mM Tris pH8.0,50 mM KCl,10 mL MgCl2)中孵育30分钟。用SensoLyte MG磷酸盐测定试剂盒测量反应释放的无机磷酸盐。在酶标板读数器上在620nm处测量吸光度。对于抑制测定,将MAT2A与FIDAS试剂在室温下孵育20分钟,然后在0.5mL反应缓冲液中与L-甲硫氨酸和ATP混合。加入冷去离子水(2mL)以停止反应并稀释样品。 MAT2A活性抑制实验:将重组人MAT2A与L-甲硫氨酸(底物)、ATP及系列稀释的FIDAS-5(0.01-10 μM)在反应缓冲液(pH 7.5)中混合,37°C孵育60分钟后,反相HPLC结合紫外检测量化SAM生成量,从剂量-反应曲线计算IC50值[1] - MAT2A结合实验:将纯化的重组MAT2A固定在传感芯片上,FIDAS-5(0.05-50 μM)以恒定流速注入,通过表面等离子体共振(SPR)分析测定结合亲和力(KD = 0.5 μM)[1] - 酶选择性实验:采用特异性底物和检测方法,评估FIDAS-5(0.01-10 μM)对MAT1A及其他代谢酶(甲硫氨酸合酶、腺苷同型半胱氨酸酶)的抑制作用,非靶点酶的抑制率<10%[1] |
| 细胞实验 |
细胞活力测定 [1]
细胞类型: LS174T 结直肠癌 (CRC) 细胞 测试浓度: 3 μM 孵育时间: 7天 实验结果:显着抑制LS174T细胞的增殖。 (SAH)水平[1]。 抗增殖实验:结肠癌细胞(HCT116、SW480、HT-29)和正常NCM460细胞以5×103个细胞/孔接种到96孔板,过夜培养。用FIDAS-5(0.01-50 μM)处理72小时,MTT法检测细胞活力,从剂量-反应曲线推导EC50/CC50值[1] - 细胞内SAM水平检测实验:HCT116细胞经FIDAS-5(0.5-2 μM)处理24小时后裂解,提取SAM并通过反相HPLC结合紫外检测量化,与溶媒对照组对比[1] - 凋亡实验:SW480细胞以2×105个细胞/孔接种到6孔板,用FIDAS-5(0.5-2 μM)处理48小时后,Annexin V-FITC/PI染色,流式细胞术量化凋亡细胞。Western blot检测活化型caspase-3、活化型PARP和Bcl-2的表达[1] - 细胞迁移和侵袭实验:HCT116细胞(2×104个细胞/孔)接种到Transwell小室(迁移实验无包被,侵袭实验包被基质胶),上室加入FIDAS-5(0.5-2 μM)。孵育24小时后,对迁移/侵袭细胞进行染色计数,计算相对于对照组的抑制百分比[1] - MAT2A信号Western blot实验:HCT116细胞经FIDAS-5(0.1-2 μM)处理24小时后,制备细胞裂解液,Western blot检测MAT2A蛋白水平,密度分析法量化条带强度[1] |
| 动物实验 |
Animal/Disease Models: 16 athymic nude mice were injected with HT29 CRC cells [1].
Doses: 20 mg/kg. Route of Administration: po (oral gavage); kg). The liver SAM levels were Dramatically higher. Dramatically diminished [1]. Routine; two-week Experimental Results: Significant inhibition of xenograft tumor growth. Colon Cancer Xenograft Efficacy Study: Female BALB/c-nu mice (6-8 weeks old, 18-22 g) were subcutaneously inoculated with 5×106 HCT116 cells. When tumors reached 100-150 mm³, mice were randomly divided into 3 groups (n=8/group): 1) Vehicle control (10% DMSO + 90% saline); 2) FIDAS-5 (10 mg/kg/day, intraperitoneal injection); 3) FIDAS-5 (20 mg/kg/day, intraperitoneal injection). Treatment continued for 21 days. Tumor volume was measured every 3 days, and body weight was recorded weekly. Mice were euthanized on day 21, and tumors were collected for SAM quantification, immunohistochemistry, and histopathological analysis[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In Vitro Cytotoxicity: Low toxicity to normal human colonic epithelial cells (NCM460, CC50 > 50 μM), resulting in a therapeutic index (EC50 colon cancer/EC50 NCM460) > 40[1]
- In Vivo Acute Toxicity: Single intraperitoneal doses up to 50 mg/kg in mice did not cause mortality or significant toxicity (e.g., lethargy, abnormal feeding behavior)[1] - Subchronic Toxicity: Mice treated with 20 mg/kg/day for 21 days showed no significant changes in hematological parameters (RBC, WBC, platelets) or liver/kidney function (ALT, AST, BUN, creatinine). No histopathological lesions were observed in major organs (liver, kidney, colon, heart)[1] |
| 参考文献 | |
| 其他信息 |
Methionine S-adenosyltransferase 2A (MAT2A) is the catalytic subunit for synthesis of S-adenosylmethionine (SAM), the principal methyl donor in many biological processes. MAT2A is up-regulated in many cancers, including liver cancer and colorectal cancer (CRC) and is a potentially important drug target. We developed a family of fluorinated N,N-dialkylaminostilbene agents, called FIDAS agents, that inhibit the proliferation of CRC cells in vitro and in vivo. Using a biotinylated FIDAS analogue, we identified the catalytic subunit of MAT2A as the direct and exclusive binding target of these FIDAS agents. MAT2B, an associated regulatory subunit of MAT2A, binds indirectly to FIDAS agents through its association with MAT2A. FIDAS agents inhibited MAT2A activity in SAM synthesis, and depletion of MAT2A by shRNAs inhibited CRC cell growth. A novel FIDAS agent delivered orally repressed CRC xenografts in athymic nude mice. These findings suggest that FIDAS analogues targeting MAT2A represent a family of novel and potentially useful agents for cancer treatment.[1]
In summary, researchers developed a novel family of stilbenes analogs, the FIDAS agents, by optimizing their anti-cancer activity. Our studies, as reported here, provide convincing evidence that the direct target of FIDAS analogs is MAT2A, specifically the catalytic subunit responsible for the synthesis of SAM. MAT2A levels are significantly increased in CRC and liver cancers, suggesting that MAT2A is a potentially interesting target for these cancers. We also analyzed the effects of FIDAS agents on other cancer cells, and found that these agents can inhibit the growth of multiple human cancer cell lines, including breast, prostate, lung, liver, carcinoid and head and neck cancer cells. Our finding that FIDAS agents affect SAM synthesis that in turn plays such a central role in numerous biological processes suggests an important mechanism that can be exploited for cancer treatment. The FIDAS agents, which uniquely block the activity of the catalytic subunit of MAT2A, are promising lead candidates and great experimental tools to study the role of MAT2A inhibition in cancer therapy. Background: FIDAS-5 is a synthetic fluorinated N,N-dialkylaminostilbene derivative identified as a selective MAT2A inhibitor for colon cancer treatment[1] - Mechanism of Action: Binds to the active site of MAT2A, inhibiting its enzymatic activity to reduce SAM synthesis. This disrupts methionine metabolism and epigenetic regulation (DNA/protein methylation), leading to colon cancer cell proliferation arrest, apoptosis, and reduced invasion[1] - Therapeutic Indication: Proposed for the treatment of colon cancer, targeting MAT2A-overexpressing colon cancer subtypes[1] - Structural Feature: Contains a fluorinated stilbene scaffold and N,N-dialkylamino moiety, which are critical for MAT2A binding affinity and selectivity. Fluorine substitution enhances metabolic stability and target binding[1] - Key Advantages: High selectivity for MAT2A over MAT1A and other metabolic enzymes; potent anti-proliferative and anti-invasive activity against colon cancer cells; favorable safety profile with low systemic toxicity[1] |
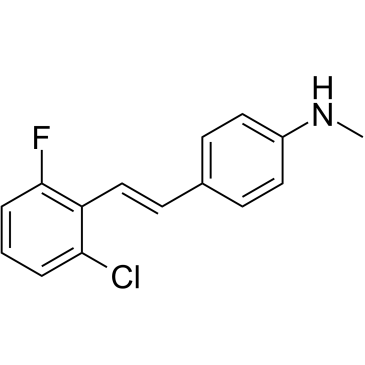
| 分子式 |
C15H13CLFN
|
|---|---|
| 分子量 |
261.721826314926
|
| 精确质量 |
261.07205
|
| CAS号 |
1391934-98-7
|
| PubChem CID |
57521314
|
| 外观&性状 |
Light yellow to yellow solid
|
| LogP |
4.9
|
| tPSA |
12Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
274
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C=CC=C(C=1/C=C/C1C=CC(=CC=1)NC)F
|
| InChi Key |
KXVXICBOMOGFMH-JXMROGBWSA-N
|
| InChi Code |
InChI=1S/C15H13ClFN/c1-18-12-8-5-11(6-9-12)7-10-13-14(16)3-2-4-15(13)17/h2-10,18H,1H3/b10-7+
|
| 化学名 |
4-[(E)-2-(2-chloro-6-fluorophenyl)ethenyl]-N-methylaniline
|
| 别名 |
FIDAS-5; 1391934-98-7; (E)-4-(2-chloro-6-fluorostyryl)-N-methylaniline; 4-[(E)-2-(2-chloro-6-fluorophenyl)ethenyl]-N-methylaniline; 4-[(1E)-2-(2-chloro-6-fluorophenyl)ethenyl]-N-methylaniline; CHEMBL3314420; SCHEMBL11895362; SCHEMBL11895364;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~477.61 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.95 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.95 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8209 mL | 19.1044 mL | 38.2088 mL | |
| 5 mM | 0.7642 mL | 3.8209 mL | 7.6418 mL | |
| 10 mM | 0.3821 mL | 1.9104 mL | 3.8209 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。