| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
| 靶点 |
DNA synthesis; Bacterial; HSV; CMV
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Floxuridine 对 PEPT1 的亲和力比相应的 5-O-单氨基酸酯前药更高。氟尿苷与亚叶酸联合对人 T 淋巴细胞白血病细胞的生长产生协同抑制作用。 Floxuridine 显着抑制 [(3)H]-肌苷和 [(3)H]-腺苷的摄取(对照的 60-70%),而其氨基酸酯前药包括 Val、Phe、Pro、Asp 和 Lys酯类表现出显着降低的抑制效力(对照的 10-30%)。 36天时,氟尿苷相对于未处理的对照细胞抑制细胞增殖超过50%,与初始细胞密度相比,细胞数量仍增加四倍。 Floxuridine 对体外人 Tenons 囊成纤维细胞的增殖具有长期影响。氟尿苷(FUDR)因其半衰期短、剂量反应曲线陡、全身清除率高、肝提取率高,是肝动脉输注(HAI)的理想药物。
|
| 体内研究 (In Vivo) |
肝转移主要由肝动脉供血。通过肝动脉给药的某些药物可以实现持续的高水平瘤内药物Floxuridine(FUDR)因其半衰期短、剂量反应曲线陡峭、全身清除率高、肝脏提取率高,是肝动脉输注(HAI)的理想药物。HAI FUDR一直显示出比单独全身化疗更高的反应率,一些研究表明它具有生存优势。HAI FUDR联合全身化疗多年来不断发展,可用于姑息治疗、新辅助治疗和辅助治疗。HAI FUDR加现代全身化疗观察到的显著反应为选定患者的切除和治愈提供了可能性。FUDR的高肝提取物限制了全身副作用。毒性包括胆道和胃肠道溃疡。[5]
HAIFloxuridine(FUDR)单独使用或与全身化疗联合使用,可导致高反应率、更长的无肝进展生存期,并提高CRC不可切除肝病的切除率。50多年来,氟丙脒一直是CRC治疗的基石。将这些药物,特别是FUDR,输送给CRC肝转移患者的一个好方法是通过HAI。现代植入式HAI泵的即时和长期并发症发生率较低。HAI FUDR联合现代全身化疗是治疗此类肝转移患者的有效方法。在辅助治疗中,HAI FUDR和全身化疗组合可以提高无病生存率和无肝病生存率。这些研究并没有着眼于总体生存率。应考虑在肝切除时放置HAI泵,并辅以HAI FUDR加全身化疗。如果肝脏疾病不可切除,并且有或没有生物制剂(如贝伐单抗或西妥昔单抗)的全身化疗试验未能使肝脏可切除,则应考虑HAI加全身化疗。HAI FUDR/Dex联合现代全身化疗在治疗结直肠癌肝转移中起着重要作用[5]。 Floxuridine (FUDR)(5’-氟脱氧尿苷,FUdR)通过与胸苷酸合酶结合,作为DNA复制的抑制剂,广泛用于治疗癌症。FUdR也经常用于秀丽隐杆线虫衰老的研究,因为通过阻断繁殖,它可以维持同步的线虫种群。在这里,研究了暴露于50μM FUdR对病理和死亡率的年龄特异性影响。报告说,在发育后期或成年早期开始接触FUdR会缩短寿命,但后期开始会延长寿命。此外,早期开始会导致衰老性肠萎缩的加剧,但会改善其他几种衰老病理(咽部退化和子宫肿瘤)。这些结果为FUdR对秀丽隐杆线虫衰老的复杂影响提供了进一步的证据,因此支持了反对将其常规用于线虫衰老研究的论点,因为它可能具有混杂作用。然而,他们也说明了FUdR对衰老的影响本身是多么有趣[6]。 |
| 酶活实验 |
水解研究。[1]
(a) 酶稳定性[1] 将汇合的Caco-2、Capan-2和AsPC-1细胞用盐水冲洗两次。用5mL pH 7.4的磷酸盐缓冲液(10mmol/L)洗涤细胞,通过超声波裂解,并在1000g下离心5分钟。使用牛血清白蛋白作为标准,用Bio-Rad DC蛋白质测定法定量蛋白质量。将蛋白质量调节至500μg/mL,在96孔板中进行水解反应。将Caco-2、AsPC-1和Capan-2细胞悬浮液(250μL)放入三个重复孔中,反应从添加底物开始,细胞在37°C下孵育120分钟。在所需的时间点,取出样品等分试样(35μL),加入150μL含有0.1%TFA的乙腈(ACN)中。用0.45μm过滤器在4°C下以1000g过滤混合物10分钟。然后通过反相HPLC分析滤液。 (b) 人血浆稳定性[1] 使用以下程序测定前药在人血浆中的稳定性。将未稀释的血浆(250μL)加入每个孔中,一式三份,并加入底物以引发在37°C下进行2小时的反应。在不同的时间点,取出等分试样(35μL),加入150μL含有0.1%TFA的ACN中。用0.45μm过滤器在4°C下以1000g过滤混合物10分钟。然后通过反相HPLC分析滤液。 (c) 化学稳定性[1] 如上所述测定前药的非酶水解,除了每个孔含有pH 7.4的磷酸盐缓冲液(10mmol/L)而不是细胞匀浆或人血浆。 (d) 胸苷磷酸化酶对Floxuridine (FUDR)/氟尿苷(FUDR)及其前药代谢的抗性[1] 通过在37°C下将所需底物(200μM)与TP(2.0 ng/μL)在磷酸盐缓冲液(pH 7.0)中孵育,评估氟尿苷及其前药在胸苷磷酸化酶(TP)存在下的稳定性。在0、1、3、5、10、30、60和120分钟时对孵育混合物的等分试样进行取样,用含有0.1%TFA的冷乙腈(ACN)淬灭,通过0.45μm膜过滤,并通过HPLC分析前药、氟尿苷和5-FU的浓度。 [3H]Gly-Sar摄取抑制[1] 将接种后9天的Caco-2细胞和接种后4天的AsPC-1和Capan-2细胞与10μmol/L的Gly-Sar(9.98μmol/L Gly-Sar和0.02μmol/L[3H]Gly-Sar)以及各种前药浓度(5-0.05mmol/L)一起孵育30分钟。用冰冷的PBS洗涤细胞三次,用10mL闪烁鸡尾酒溶解,并通过闪烁计数测定细胞相关的放射性量。使用非线性数据拟合(GraphPad Prism 3.0版)确定IC50值。 交通研究[1] Caco-2细胞单层在胶原包被的聚四氟乙烯膜上生长21至24天,Capan-2细胞单层则在相同类型的膜上生长14天。监测跨上皮电阻(TEER),研究中使用Caco-2为240−280Ω/cm2,Capan-2为380−420Ω/cm2(两个细胞的总面积为4.67 cm2)。分别用MES(pH 6.0)和HEPES(pH 7.4)洗涤经口插入物的根尖侧和基底外侧。将新鲜的MES和HEPES缓冲液重新施加到transwell插入物上,并在37°C下孵育15分钟。将新鲜制备的0.1 mM MES缓冲液药物溶液(共1.5 mL)放入供体室,接收器室填充HEPES缓冲溶液(共2.5 mL)。在37°C下,以15、30、45、60、75、90和120分钟的时间间隔从接收器室(200μL)中取样2小时,并用等体积的新鲜HEPES缓冲物替换,以保持接收器室中的沉降条件。所有样品立即用0.1%TFA酸化,并通过反相HPLC进行分析。 开发了抗病毒和抗癌核苷药物的氨基酸酯前药,以提高口服生物利用度或降低全身毒性。研究人员研究了从肠道克隆的人浓缩核苷转运蛋白(hCNT2)与各种氨基酸酯前药Floxuridine (FUDR)/氟尿苷(FUdR)和5,6-二氯-2-溴-1-β-D-呋喃核糖基苯并咪唑(BDCRB)的相互作用。在瞬时表达肠hCNT2的U251细胞中,测量了Na(+)依赖性摄取[(3)H]-肌苷和[(3-H]-腺苷。FUdR显著抑制了[(3)H]-肌苷和[(3”H]-腺苷的摄取(对照组的60-70%),而其氨基酸酯前药包括Val、Phe、Pro、Asp和Lys酯,其抑制效力显著降低(对照组为10-30%)。另一方面,BDCRB及其氨基酸前体药物显著抑制了[(3)H]-肌苷和[(3-H]-腺苷的摄取。BDCRB的缬氨酸、苯丙氨酸和前酯前药显示出与母体化合物BDCRB相似的抑制能力(腺苷为80-90%,肌苷为60-80%)。氨基酸附着位点(3'-和5'-单酯)和立体化学(L-和D-氨基酸酯)对[(3)H]-肌苷和[(3”H]-腺苷的摄取没有显著影响。这些结果表明,与FUdR相比,hCNT2与BDCRB及其氨基酸前体药物有良好的相互作用,BDCRB的中性氨基酸酯对这种转运蛋白具有很高的亲和力。因此,肠道hCNT2可能是调节BDCRB前药口服药代动力学的靶转运蛋白[3]。 |
| 细胞实验 |
细胞系:卵巢癌细胞
浓度:0-25 μM 孵育时间:4、8、24 小时 结果:PARP 抑制剂增强了敏感性。 细胞增殖试验[1] 用AsPC-1和Capan-2细胞系进行细胞增殖研究。将细胞以每孔125000个细胞的速度接种到96孔板上,并在加入药物溶液之前使其附着/生长24小时。移除培养基(RPMI-1640+10%胎牛血清),用无菌pH 6.0摄取缓冲液轻轻洗涤细胞一次氟尿苷(FUDR)和氟尿苷前药在pH 6.0的摄取缓冲液中从4到0.25 mmol/L连续稀释2倍。单独使用缓冲液作为100%存活率对照。去除洗涤缓冲液,每孔加入25μL药物溶液,在细胞培养箱中与AsPC-1细胞在37°C下孵育2小时,与Capan-2细胞孵育4小时。在此时间段后,取出药物溶液,用无菌摄取缓冲液轻轻洗涤细胞两次。洗涤后,将新鲜的培养基加入每个孔中,让细胞恢复24小时,然后通过2,3-双[2-甲氧基-4-硝基-5-磺基苯基]-2H-四唑-5-甲酰苯胺内盐(XTT)测定评估细胞存活率。将含有XTT(1 mg/mL)的无菌RPMI-1640混合物(30μL)加入细胞中,不含酚红和吩嗪甲硫酸盐(无菌PBS中的N-甲基二苯并吡嗪甲硫酸盐,0.383 mg/mL)试剂,在37°C下孵育1小时,然后读取450 nm处的吸光度。GI50值是使用GraphPad Prism 3.0版通过非线性数据拟合计算的。 甲酰四氢叶酸(LV)联合5-氟尿嘧啶(FUra)或Floxuridine (FUDR) 对人T淋巴细胞白血病细胞(CCRF-CEM)生长的抑制作用被确定为时间、剂量和暴露顺序的函数。将呈指数增长的CCRF-CEM细胞暴露于LV(1-100微M)4小时,并在最后2小时暴露于FUra(100微米)或FdUrd(0.5微米),对细胞生长产生协同抑制作用。协同作用取决于左心室剂量(100大于10大于1微M),在0.1微M时不会发生。FUra和LV组合没有观察到协同作用对序列的明显依赖性。对于LV和FdUrd组合,协同作用取决于暴露顺序(LV+FdUrd和LV----FdUrd是协同作用的,但FdUrd-----LV不是)。经药物处理后加入的胸苷(0.1μM)显著地保护了CCRF-CEM细胞免受LV-FUra的细胞毒性。伴随的次黄嘌呤(100μM)仅部分保护CCRF-CEM细胞免受这种组合的毒性。这些结果与LV增强氟嘧啶细胞毒性的机制是增强胸苷酸合酶和5-氟脱氧尿苷酸之间的复合物形成的假设是一致的,这可能是LV产生的5,10-亚甲基四氢叶酸细胞内水平增加的结果。此外,在高水平叶酸辅酶存在下,这种复合物的稳定性增强可能有助于观察到的协同作用。这些数据也为使用FUra,特别是FdUrd和LV治疗男性淋巴系统恶性肿瘤提供了理论基础。[2] 将增殖的人Tenon囊成纤维细胞暴露于不同浓度的氟尿嘧啶、氟尿嘧啶(FUDR)和丝裂霉素中5分钟。与未处理的对照细胞相比,所有三种药物的高浓度对细胞增殖和形态特征的影响延长了36天。最高浓度的氟尿苷(15000微克/毫升)和丝裂霉素(1000毫克/毫升)都有明显的杀菌作用,将细胞数量减少到初始细胞密度以下。相比之下,尽管最高浓度的氟尿嘧啶(25000微克/毫升)在36天时相对于未处理的对照细胞抑制了50%以上的细胞增殖,但细胞数量仍比初始细胞密度增加了四倍。这些结果表明,用高浓度的这些药物进行5分钟的治疗,对体外培养的人Tenon囊成纤维细胞的增殖有延长作用。在手术时使用高浓度这些药物的单剂量方案可能会达到与涉及重复应用的方案类似的结果[4]。 |
| 动物实验 |
C57BL/6 mice injected with S. aureus
0.5-1.25 mg/kg once per day for 7 days or single dose To examine the effects of Floxuridine (FUDR) on worms, drug was added topically onto a 2-day old bacterial lawn to a final concentration of 50 μM. Worms were transferred from untreated NGM plates at L4, or day 1, day 2 or day 3 of adulthood. [6] Lifespan assays[6] Gravid adults were cultured on fresh NGM plates to lay eggs, and two days later L4 larvae were picked to new plates for lifespan measurements. Trials were conducted at 20 °C for N2 strains. Temperature sensitive sterile glp-4(bn2) mutants were cultured at 15 °C until L4 and then transferred to 25 °C. Nematodes were transferred daily during the reproductive period to avoid confusion with progeny. Population cohorts were scored for mortality every other day until the death of the last worm. Deaths due to internal hatching of larvae or rupture were censored. The L4 stage was recorded as day 0 for the trials. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Floxuridine can be excreted as unchanged drug, urea, fluorouracil, a-fluoro-bureidopropionic acid, dihydrofluorouracil, a-fluoro-b-guanidopropionic acid and a-fluoro-b-alanine via the kidneys. Floxuridine may also be excreted as respiratory carbon dioxide. ... Floxuridine /is/ ... administered parenterally, since absorption after ingestion ... is unpredictable and incomplete. It is not known whether floxuridine is distributed into milk. Some /Floxuridine/ crosses the blood-brain barrier; active metabolites are localized intracellularly. Elimination /is/ respiratory (as carbon dioxide), about 60%. Renal /elimination accounts for/ 10 to 13% (as unchanged drug and metabolites). Metabolism / Metabolites Hepatic. Biotransformation /is/ hepatic and in tissues, extensive, to the monophosphate derivative and fluorouracil; after continuous intra-arterial infusion, conversion to the monophosphate derivative is enhanced; largely converted to fluorouracil after rapid intravenous or intra-arterial injection. Following infusion of small doses of floxuridine, most of the drug appears to be anabolized to FUDR-MP, the active metabolite of the drug. When single doses are administered rapidly, floxuridine is apparently rapidly catabolized to fluorouracil. Floxuridine and fluorouracil are metabolized in the liver. Metabolic degradation of floxuridine is less when the drug is given by continuous infusion than when given by single injections. The drug is excreted intact and as urea, fluorouracil, a-fluoro-ß-ureidopropionic acid, dihydrofluorouracil, alpha-fluoro-beta-guanidopropionic acid, and alpha-fluoro-beta-alanine in the urine and as respiratory carbon dioxide. Metabolic degradation occurs, particularly in the liver. Floxuridine is converted by thymidine or deoxyuridine phosphorylases into 5-fluorouracil. 5-Fluorouracil is inactivated by reduction of the pyrimidine ring; this reaction is carried out by dihydrouracil dehydrogenase, which is found in liver, intestinal mucosa, and other tissues. Inherited deficiency of this enzyme leads to greatly increased sensitivity to the drug. The product of this reaction, 5-fluoro-5,6-dihydrouracil is ultimately degraded to alpha-fluoro-beta-alanine ... . |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Serum aminotransferase elevations occur in a high proportion of patients given floxuridine by infusion into the hepatic artery, the reported rates ranging from 25% to 100%. These elevations are generally mild to moderate in severity and resolve with stopping therapy. "Chemical hepatitis," however, not infrequently is a cause of dose modification or delay in cycles of treatment. In addition, prolonged or repeated hepatic arterial infusions of FUDR can cause acalculous cholecystitis and multiple biliary strictures that can cause jaundice and a chronic sclerosing cholangitis-like syndrome. Between 5% and 25% of patients treated with hepatic arterial infusions of FUDR will develop symptomatic biliary strictures with pain and jaundice. These typically arise after 2 to 6 months of therapy, but can appear later, even more than a year after initiating FUDR therapy. The biliary strictures typically affect central bile ducts in the area of the porta hepatis, generally in and around the bifurcation of the common hepatic duct. Similar inflammation and fibrosis account for the acalculous cholecystitis that can occur with FUDR therapy, but which can be avoided by cholecystectomy at the time of hepatic resection of metastases or placement of the intraarterial infusion pump. The biliary strictures generally improve with stopping therapy, but can progress or require endoscopic or surgical intervention. Deaths from progressive biliary strictures and cholestatic liver injury have been described and can be a major cause of death among survivors of this metastatic tumor. The frequency of biliary strictures after FUDR therapy may be decreased by concurrent administration of dexamethasone and avoided by monitoring with hepatic and biliary imaging. However, the many complications of hepatic arterial infusion chemotherapy have decreased enthusiasm for this therapy, particularly with newer, more potent systemic antineoplastic agents. Likelihood score: A (well known cause of clinically apparent liver and biliary injury). Interactions Leukopenic and/or thrombocytopenic effects of floxuridine may be increased with concurrent or recent therapy /with blood dyscrasia-causing medications/ if these medications cause the same effects; dosage adjustment of floxuridine, if necessary, should be based on blood counts. Additive bone marrow depression may occur; dosage reduction may be required when two or more bone marrow depressants, including radiation, are used concurrently or consecutively /with floxuridine/. Because normal defense mechanisms may be suppressed by floxuridine therapy, the patients antibody response to killed virus vaccines may be decreased. The interval between discontinuation of medications that cause immunosuppression and restoration of the patients ability to respond to the vaccine depends on the intensity and type of immunosuppression-causing medication used, the underlying disease, and other factors; estimates vary from 3 months to 1 year. Because normal defense mechanisms may be suppressed by floxuridine therapy, concurrent use with a live virus vaccine may potentiate the replication of the vaccine virus, may increase the side/adverse effects of the vaccine virus, and/or may decrease the patients antibody repose to the vaccine; immunization of these patients should be undertaken only with extreme caution after careful review of the patient's hematological status and only with the knowledge and consent of the physician managing the floxuridine therapy. The interval between discontinuation of medications that cause immunosuppression and restoration of the patients ability to respond to the vaccine depends on the intensity and type of immunosuppression-causing medication used, the underlying disease, and other factors; estimates vary from 3 months to 1 year. Immunization with oral poliovirus vaccine should also be postponed in persons in close contact with the patient, especially family members. Toxicity of HAI FUDR [5] The toxicity of HAI can be mechanical, chemical, or a combination of both. Surgically implantable pumps have low complication rates. Allen and colleagues reported on HAI pump complications in 544 patients throughout the course of treatment. Complications within the first 30 days of placement were more likely to be catheter occlusions or arterial thromboses and less likely to be salvaged. Overall rates of pump failure were low being 9% at 1 year and 16% at 2 years. The overall pump complication rate was 22%, and the majority of these complications were salvaged with 80% remaining functional for at least 2 years. All patients have a nuclear medicine macro-aggregated albumin scan before the pump is used to assess whether the liver is being perfused or if there is extrahepatic perfusion via side branches of the gastroduodenal artery. If there is misperfusion to the stomach or duodenum, ulceration or diarrhea can result. In contrast to systemic chemotherapy, myelosuppression, nausea, and vomiting do not occur with HAI FUDR. Hepatotoxicity from HAI depends on the drugs being used and the duration of treatment. The hepatic artery supplies the bile ducts, and therefore toxicity of HAI FUDR can be biliary. Elevation in liver enzymes or bilirubin is the most common toxicity associated with HAI therapy, occurring in 42% of patients in the randomized trials for unresectable liver disease reported above. Increase in transaminase levels is not uncommon (up to 70% of cases) and can be an early sign of biliary damage. Increases in bilirubin and alkaline phosphatase are more serious. Up to 29% of cases before the addition of Dex to the pump developed strictures of the bile ducts (biliary sclerosis) (ref. 38). In the adjuvant pump studies at our institution, a larger than twofold increase in alkaline phosphatase was seen in 27% to 43% of cases. An increase in bilirubin greater than 3.0 mg/dL was seen in 6% to 19% of cases with biliary stents required in 3% to 8% of cases. Transaminases increased by 37% to 59%. In the recently updated study by Kemeny and colleagues, HAI FUDR/Dex was combined with oxaliplatin and irinotecan over a 5-week cycle. Toxicities during the first two cycles were: grade 3 diarrhea (33%), grade 3/4 alkaline phosphatase (15% and 11%, respectively), grade 3/4 AST (19%), grade 3 bilirubin (4%), and grade 3/4 neutropenia (19% and 4%, respectively). Late toxicity (after the first two cycles) included grade 3 diarrhea (7%), grade 3 or 4 alkaline phosphatase (22% and 11%, respectively), grade 3/4 AST (7%), grade 3/4 neutropenia (15% and 11%, respectively), and neurotoxicity (19%). Thus an algorithm for dose reductions based on liver blood tests has been devised, and FUDR can be dose adjusted accordingly (see Table 3). As mentioned above, the addition of Dex to FUDR reduces biliary toxicity. HAI FUDR/Dex is administered every month (if enzymes are normal). The pump continuously infuses drug, and after 2 weeks the reservoir is emptied and then refilled with heparin saline or glycerol, which then infuses over a further 2 weeks. If a patient develops an elevated bilirubin, chemotherapy is held, and Dex with heparinized saline is placed in the pump. If the bilirubin still does not normalize, then an ERCP can be done to evaluate for focal strictures that may respond to dilatation. When the pump is not in use, glycerol is inserted every 6 to 8 weeks to keep the catheter patent. If the pump is no longer required it can be surgically removed by a small incision and the catheter in the hepatic artery is cut off from the pump and left in place. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antimetabolites, Antineoplastic; Antiviral Agents Floxuridine, given by continuous regional intra-arterial infusion, is indicated for palliative management of colorectal carcinoma metastatic to the liver that has not responded to other treatment. Floxuridine is most useful when the disease has not extended beyond an area capable of infusion via a single artery. /Included in US product labeling/ Floxuridine also is indicated for carcinoma of the ovary and kidney not responsive to other antimetabolites. /Not Include in the US product label/ Floxuridine has also been used for carcinoma of the breast, ovary, cervix, urinary bladder, kidney, and prostate not responsive to other antimetabolites. /NOT included in US product labeling/ Drug Warnings Floxuridine is metabolized to fluorouracil, but the full spectrum of fluorouracil toxicity is not expected with floxuridine because of regional administration of the drug by intra-arterial infusion. However, the possibility of typical adverse effects of fluorouracil during floxuridine therapy should be considered. Nausea, vomiting, and diarrhea are common adverse effects; anorexia, cramps, and pain also may occur. Stomatitis is one of the most common signs of specific toxicity. Enteritis occurs frequently and duodenal ulcer, duodenitis, gastritis, gastroenteritis, glossitis, GI bleeding, and pharyngitis also have been reported. Leukopenia and anemia occur commonly with floxuridine therapy; thrombocytopenia also may occur. The patient's hematologic status must be carefully monitored. Pancytopenia and agranulocytosis have been reported in patients receiving fluorouracil; because of its pharmacologic similarity, these adverse hematologic effects might occur in patients receiving floxuridine. For more Drug Warnings (Complete) data for FLOXURIDINE (25 total), please visit the HSDB record page. Pharmacodynamics Floxuridine is an anti-metabolite or a pyrimidine analog that works by disrupting the process S-phase of cell division, selectively targeting rapidly dividing cells. Due to the structural similarities, antimetabolites act as pyrimidine-like molecules and prevent normal pyrimidines from being incorporated into DNA. After successful biotransformation, floxuridine is converted into an active component, flurouracil, which blocks the enzyme which converts cytosine nucleosides into the deoxy derivative. Flurouracil also physically prevents the incorporation of thymidine nucleotides into the DNA strand by taking their place, further preventing DNA synthesis. Dipeptide monoester prodrugs of floxuridine were synthesized, and their chemical stability in buffers, resistance to glycosidic bond metabolism, affinity for PEPT1, enzymatic activation and permeability in cancer cells were determined and compared to those of mono amino acid monoester floxuridine prodrugs. Prodrugs containing glycyl moieties were the least stable in pH 7.4 buffer ( t 1/2 < 100 min). The activation of all floxuridine prodrugs was 2- to 30-fold faster in cell homogenates than their hydrolysis in buffer, suggesting enzymatic action. The enzymatic activation of dipeptide monoester prodrugs containing aromatic promoieties in cell homogenates was 5- to 20-fold slower than that of other dipeptide and most mono amino acid monoester prodrugs ( t 1/2 approximately 40 to 100 min). All prodrugs exhibited enhanced resistance to glycosidic bond metabolism by thymidine phosphorylase compared to parent floxuridine. In general, the 5'-O-dipeptide monoester floxuridine prodrugs exhibited higher affinity for PEPT1 than the corresponding 5'-O-mono amino acid ester prodrugs. The permeability of dipeptide monoester prodrugs across Caco-2 and Capan-2 monolayers was 2- to 4-fold higher than the corresponding mono amino acid ester prodrug. Cell proliferation assays in AsPC-1 and Capan-2 pancreatic ductal cell lines indicated that the dipeptide monoester prodrugs were equally as potent as mono amino acid prodrugs. The transport and enzymatic profiles of 5'- l-phenylalanyl- l-tyrosyl-floxuridine, 5'- l-phenylalanyl- l-glycyl-floxuridine, and 5'- l-isoleucyl- l-glycyl-floxuridine suggest their potential for increased oral uptake, delayed enzymatic bioconversion and enhanced resistance to metabolism to 5-fluorouracil, as well as enhanced uptake and cytotoxic activity in cancer cells, attributes that would facilitate prolonged systemic circulation for enhanced therapeutic action. [1] Floxuridine is a pyrimidine 2'-deoxyribonucleoside compound having 5-fluorouracil as the nucleobase; used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract. It has a role as an antineoplastic agent, an antimetabolite, an antiviral drug and a radiosensitizing agent. It is a pyrimidine 2'-deoxyribonucleoside, an organofluorine compound and a nucleoside analogue. An antineoplastic antimetabolite that is metabolized to fluorouracil when administered by rapid injection. Floxuridine is available as a sterile, nonpyrogenic, lyophilized powder for reconstitution. When administered by slow, continuous, intra-arterial infusion, it is converted to floxuridine monophosphate. It has been used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract. Floxuridine is an Antimetabolite. Floxuridine (FUDR) is a pyrimidine analogue used as an antineoplastic agent, usually as a continuous hepatic arterial infusion to treat hepatic metastases from colon cancer. Intraarterial floxuridine is associated with a very high rate of serum enzyme and bilirubin elevations during therapy, and with frequent biliary damage that can result in a secondary sclerosing cholangitis, which can be severe and lead to cirrhosis. Floxuridine is a fluorinated pyrimidine monophosphate analogue of 5-fluoro-2'-deoxyuridine-5'-phosphate (FUDR-MP) with antineoplastic activity. As an antimetabolite, floxuridine inhibits thymidylate synthase, resulting in disruption of DNA synthesis and cytotoxicity. This agent is also metabolized to fluorouracil and other metabolites that can be incorporated into RNA and inhibit the utilization of preformed uracil in RNA synthesis. (NCI04) FLOXURIDINE is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 1970 and is indicated for cancer and neoplasm and has 17 investigational indications. This drug has a black box warning from the FDA. An antineoplastic antimetabolite that is metabolized to fluorouracil when administered by rapid injection; when administered by slow, continuous, intra-arterial infusion, it is converted to floxuridine monophosphate. It has been used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract. |
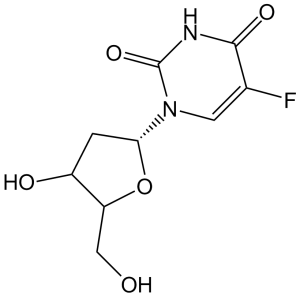
| 分子式 |
C9H11FN2O5
|
|
|---|---|---|
| 分子量 |
246.19
|
|
| 精确质量 |
246.065
|
|
| 元素分析 |
C, 43.91; H, 4.50; F, 7.72; N, 11.38; O, 32.49
|
|
| CAS号 |
50-91-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5790
|
|
| 外观&性状 |
White powder
|
|
| 密度 |
1.8±0.1 g/cm3
|
|
| 沸点 |
483.0±55.0 °C at 760 mmHg
|
|
| 熔点 |
148 °C(lit.)
|
|
| 闪点 |
245.9±31.5 °C
|
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
|
| 折射率 |
1.676
|
|
| LogP |
-1.22
|
|
| tPSA |
104.55
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
386
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
FC1C(N([H])C(N(C=1[H])[C@@]1([H])C([H])([H])[C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])=O)=O
|
|
| InChi Key |
ODKNJVUHOIMIIZ-RRKCRQDMSA-N
|
|
| InChi Code |
InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
|
|
| 化学名 |
5-fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (8.45 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (8.45 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (8.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (406.19 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0619 mL | 20.3095 mL | 40.6190 mL | |
| 5 mM | 0.8124 mL | 4.0619 mL | 8.1238 mL | |
| 10 mM | 0.4062 mL | 2.0310 mL | 4.0619 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00492999 | Active Recruiting |
Drug: floxuridine Drug: fluorouracil |
Colorectal Cancer Metastatic Cancer |
Memorial Sloan Kettering Cancer Center |
May 2007 | Phase 2 |
| NCT00410956 | Active Recruiting |
Drug: floxuridine Drug: dexamethasone |
Liver Cancer | Memorial Sloan Kettering Cancer Center |
May 2007 | Phase 2 |
| NCT01862315 | Active Recruiting |
Drug: Floxuridine (FUDR) Drug: dexamethasone |
Cholangiolar Carcinoma Cholangiocellular Carcinoma |
Memorial Sloan Kettering Cancer Center |
May 2013 | Phase 2 |
| NCT00059930 | Active Recruiting |
Drug: floxuridine Drug: fluorouracil |
Colorectal Cancer Metastatic Cancer |
Memorial Sloan Kettering Cancer Center |
January 2003 | Phase 1 |
| NCT03693807 | Active Recruiting |
Drug: Floxuridine (FUDR) Drug: Gemcitabine |
Febrile Neutropenia Cholangiocarcinoma |
Memorial Sloan Kettering Cancer Center |
October 18, 2018 | Phase 2 |
|