| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and virtually completely absorbed following oral administration. Bioavailability 78% to 89%. Flucytosine is excreted via the kidneys by means of glomerular filtration without significant tubular reabsorption. A small portion of the dose is excreted in the feces. Flucytosine is rapidly and well absorbed from the GI tract, with plasma levels peaking in 1-2 hr in animals that have received the drug for several days. The drug is widely distributed in the body, with a volume of distribution approximating the total body water. Flucytosine is minimally bound to plasma proteins. There is excellent penetration into body fluids such as the CSF, synovial fluids, and aqueous humor. Flucytosine is rapidly and almost completed absorbed from the GI tract. Bioavailability is 78-89% following oral administration. Food decreases the rate, but not the extent, of absorption. In a limited number of neonates receiving oral flucytosine in a dosage of 25, 50, or 100 mg/kg daily for the treatment of systemic candidiasis, median peak serum concentrations after 5 days of treatment were 19.6, 27.7, and 83.9 ug/mL, respectively, and the mean time to peak concentrations was 2.5 hours. There was considerable interindividual variation in serum concentrations, which did not correlate with gestational age, and some neonates had serum flucytosine concentrations greater than 100 ug/mL. In patients with normal renal function, peak serum flucytosine concentrations of 30-40 mcg/mL are reached within 2 hours following a single 2-g oral dose. In other studies in patients with normal renal function receiving a 6-week regimen of oral flucytosine (150 mg/kg daily given in divided doses every 6 hours) and concomitant IV amphotericin B, mean serum concentrations of flucytosine 1-2 hours after a dose were approximately 70-80 ug/mL. For more Absorption, Distribution and Excretion (Complete) data for Flucytosine (8 total), please visit the HSDB record page. Metabolism / Metabolites Flucytosine is deaminated, possibly by gut bacteria or by the fungal targets, to 5-fluorouracil, the active metabolite. Flucytosine may be fungistatic or fungicidal in action depending on the concentration of the drug. Two possible mechanisms of action have been identified for flucytosine. Flucytosine appears to enter fungal cells via the action of fungal-specific cytosine permease. Inside the cell, flucytosine is converted into fluorouracil (5-FU) by cytosine deaminase and then after several intermediate steps is converted into 5-fluorouridine triphosphate (FUTP). FUTP is incorporated into fungal RNA and interferes with protein synthesis. Flucytosine also appears to be converted to 5-fluorodeoxyuridine monophosphate, which noncompetitively inhibits thymidylate synthetase and interferes with DNA synthesis. Flucytosine does not appear to have antineoplastic activity. The aim of this study is to investigate whether fluorouracil (5-FU) could be responsible for bone-marrow depression occurring in fluorocytosine (5-FC) treated patients. Six 5-FC treated patients were included in this pilot study. Toxicity was monitored by means of thrombocyte and leucocyte counts. 5-FC and 5-FU serum levels were measured using a high-performance liquid chromatography (HPLC) assay that allows simultaneous determination of both compounds. The amounts of 5-FU in the 34 available serum samples remained below the limit of quantitation (< 0.05 mg/L), whereas 5-FC levels could be detected in all samples. Instead, low levels of the 5-FU catabolite alpha-fluoro-beta-alanine (FBAL) were detected in several of the investigated serum samples. In case of three patients thrombocyte counts remained within the normal range during 5-FC treatment, whereas one patient developed thrombocytopenia (50 x 10(9) thrombocytes/L) during therapy. Furthermore, one patient developed leucocytopenia (2.6 x 10(9) leucocytes/L) during 5-FC therapy, whereas the remaining five patients were suffering from leucocytosis prior to 5-FC therapy. In conclusion, we found nondetectable 5-FU serum concentrations (< 0.05 mg/L) in ICU patients treated with intravenous 5-FC, making it unlikely that 5-FC-associated toxicity results from 5-FU exposure in patients receiving intravenous 5-FC therapy. These findings may be explained by the fact that our patients received 5-FC intravenously instead of orally, therefore not allowing active conversion of 5-FC to 5-FU by the human intestinal microflora. /5-Fluorouracil/ A gas chromatographic-mass spectrometric method for detecting 5-fluorouracil (5-FU) in serum at concentrations as low as 10 ng/mL was used to determine to what extent 5-FU was present in the serum of patients taking oral 5-fluorocytosine (5-FC). Preliminary studies in two patients and two healthy volunteers given an initial 2-g oral dose of 5-FC demonstrated sustained serum 5-FU levels (>100 ng/mL) during the 5 hr after ingestion of drug. Pharmaceutical preparations of 5-FC used in these studies were shown to be insignificantly contaminated with 5-FU (<0.03%), suggesting in vivo conversion of 5-FC to 5-FU had occurred. Serum samples from seven patients with cryptococcal meningitis treated with amphotericin B and 5-FC were examined for 5-FU. Five of these patients had experienced hematological or other toxicity attributed to 5-FC at some time during the course of therapy. Of 41 serum samples, 20 were observed to have 5-FU levels greater than 1,000 ng/mL in the range observed with cancer chemotherapeutic doses of 5-FU known to be associated with hematological toxicity. It is concluded that conversion of 5-FC to 5-FU occurs in humans and furthermore that 5-FU may account for some of the toxicity observed with 5-FC. /5-Fluorouracil/ Metabolism of 5-fluorocytosine-6-14C (5-FC) was studied in mice, rats, rabbits and dogs after oral and subcutaneous, single and repeated administration. In the urines of all species, intact 5-FC accounted for more than 90% of the total radioactivity at any time of the various treatment schedules. The average proportion of the urinary metabolites was around 5% in dogs, 3% in rabbits, 2.5% in rats, and 2% in mice of the total radioactivity. At repeated dosage, there was an increase of metabolites in mice but a decrease in rats treated subcutaneously. Neither increase nor decrease was observed in rabbits (treated orally) and dogs. Two metabolites were identified, alpha-fluoro-beta-ureido-propionic acid (FUPA) and alpha-fluoro-beta-alanine, the latter occurring mainly after oral treatment. These compounds represent probably that part of 5-FC which was deaminated to 5-fluorouracil (5-FU) or directly to 5-fluorodihydrouracil. FUPA was the only metabolite found in the urines collected from 4 out of 5 human volunteers during the first 12 h after single oral administration of 3.5 g of the radiolabelled drug. Its maximum proportion was 1.1% of the total radioactivity. No metabolites were detected in the urine neither of the 5th volunteer nor in those of 3 mycosis patients who were given the radioactive dose after they had received regular chemotherapy with unlabelled 5-FC (150 mg/kg/day) for at least 2 weeks. The sensitivity threshold of the method was 0.1-0.4% of the total radioactivity. One of the patients had developed thrombocytopenia which was probably due to 5-FC chemotherapy. The symptoms of 5-FC intolerance were in most of the examined species similar to those observed with 5-FU [9]. However, no quantitative correlation between proportion of metabolites and 5-FC toxicity is apparent except that man is the species in which both metabolism and toxicity are the lowest. It has not been proved yet that 5-FC intolerance occurring in a small percentage of patients receiving 5-FC chemotherapy (mainly leukopenia, thrombocytopenia) results in fact from conversion to 5-FU. For more Metabolism/Metabolites (Complete) data for Flucytosine (6 total), please visit the HSDB record page. Flucytosine is deaminated, possibly by gut bacteria or by the fungal targets, to 5-fluorouracil, the active metabolite. Route of Elimination: Flucytosine is excreted via the kidneys by means of glomerular filtration without significant tubular reabsorption. A small portion of the dose is excreted in the feces. Half Life: 2.4 to 4.8 hours. Biological Half-Life 2.4 to 4.8 hours. In a limited number of infants, the median half-life of flucytosine was 7.4 hours. The half-life of flucytosine is prolonged in patients with renal insufficiency; the average half-life in nephrectomized or anuric patients was 85 hours (range: 29.9 to 250 hours). A linear correlation was found between the elimination rate constant of flucytosine and creatinine clearance. The elimination half-life of flucytosine has been variously reported to be 2.4-6 hours in patients with normal renal function, 6-14 hours in patients with creatinine clearances of 40 mL/minute, 12-15 hours in patients with creatinine clearances of 20 mL/minute, 21-27 hours in patients with creatinine clearances of 10 mL/minute, and 30-250 hours in patients with creatinine clearances less than 10 mL/minute. Half-lives up to 1160 hours have been reported in a few patients with creatinine clearances less than 2 mL/minute. Some clinicians have suggested that the half-life of flucytosine in hours is approximately 5 or 6 times the serum creatinine concentration in mg/dL. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Flucytosine is a solid. It is used as antifungal agent and antimetabolite. Flucytosine Capsules are indicated only in the treatment of serious infections caused by susceptible strains of Candida and/or Cryptococcus. HUMAN STUDIES: It is reasonable to expect that overdosage may produce pronounced manifestations of the known clinical adverse reactions. Prolonged serum concentrations in excess of 100 ug/mL may be associated with an increased incidence of toxicity, especially gastrointestinal (diarrhea, nausea, vomiting), hematologic (leukopenia, thrombocytopenia) and hepatic (hepatitis). A case of urinary crystalluria was reported in a patient during treatment with flucytosine. Flucytosine-associated diarrhea has been described in 6%-10% of patients receiving the drug. A potentially fatal ulcerating enterocolitis has been reported in 4 patients. ANIMAL STUDIES: In mice, 400 mg/kg/day of flucytosine administered on days 7 to 13 of gestation was associated with a low incidence of cleft palate that was not statistically significant. Flucytosine was not teratogenic in rabbits up to a dose of 100 mg/kg/day administered on days 6 to 18 of gestation. Flucytosine was shown to be teratogenic (vertebral fusions) in the rat at doses of 40 mg/kg/day administered on days 7 to 13 of gestation. At higher doses (700 mg/kg/day) administered on days 9 to 12 of gestation), cleft lip and palate and micrognathia were reported. The in utero treatment had no adverse effect on the fertility or reproductive performance of the offspring in mice. The mutagenic potential of flucytosine was evaluated in Ames-type studies with five different mutants of Salmonella typhimurium and no mutagenicity was detected in the presence or absence of activating enzymes. Flucytosine was nonmutagenic in three different repair assay systems. Although the exact mode of action is unknown, it has been proposed that flucytosine acts directly on fungal organisms by competitive inhibition of purine and pyrimidine uptake and indirectly by intracellular metabolism to 5-fluorouracil. Flucytosine enters the fungal cell via cytosine permease; thus, flucytosine is metabolized to 5-fluorouracil within fungal organisms. The 5-fluorouracil is extensively incorporated into fungal RNA and inhibits synthesis of both DNA and RNA. The result is unbalanced growth and death of the fungal organism. It also appears to be an inhibitor of fungal thymidylate synthase. Hepatotoxicity Transient mild-to-moderate elevations in serum aminotransferase or alkaline phosphatase levels occur in up to 41% of patients treated with flucytosine. The enzyme abnormalities are usually asymptomatic and resolve with stopping flucytosine, and sometimes even with its continuation. Clinically apparent hepatotoxicity is very rare. Instances of acute liver injury and hepatic failure have been mentioned in clinical trials of flucytosine therapy, but few details were provided and no convincing case reports of acute hepatic injury with jaundice have been published. Likelihood score: D (possible rare cause of clinically apparent liver injury. Protein Binding 28-31% Toxicity Data LD50: 15 gm/kg (Oral, Rat) (A308) Interactions Invasive fungal infection is a well-known cause of morbidity and mortality in immunocompromised patients. In this study we aimed to evaluate the hepatotoxicity induced by combined therapy of flucytosine and amphotericin B, at three different doses administered to mice for 14 days: 50 mg/kg flucytosine and 300 ug/kg amphotericin B; 100 mg/kg flucytosine and 600 ug/kg amphotericin B; 150 mg/kg flucytosine and 900 ug/kg amphotericin B. Liver injuries were evaluated by analysis of optic and electron microscopy samples, changes in TNF-alpha, IL-6, and NF-kappaB inflammation markers levels of expression, and evaluation of mRNA profiles. Histological and ultrastructural analysis revealed an increase in parenchymal and portal inflammation in mice and Kupffer cells activation. Combined antifungal treatment stimulated activation of an inflammatory pathway, demonstrated by a significant dose-dependent increase of TNF-alpha and IL-6 immunoreactivity, together with mRNA upregulation. Also, NF-kappaB was activated, as suggested by the high levels found in hepatic tissue and upregulation of target genes. Our results suggest that antifungal combined therapy exerts a synergistic inflammatory activation in a dose-dependent manner, through NF-kappaB pathway, which promotes an inflammatory cascade during inflammation. The use of combined antifungal therapy needs to be dose limiting due to the associated risk of liver injury, especially for those patients with hepatic dysfunction. In some in vitro studies, the combination of flucytosine and amphotericin B resulted in synergistic inhibition of strains of Cryptococcus neoformans, Candida albicans, and C. tropicalis. The suggested mechanism of this synergism is that the binding of amphotericin B to sterols in cell membranes increases the permeability of the cytoplasmic membrane, thus allowing greater penetration of flucytosine into the fungal cell. However, in a study evaluating the antifungal effects of the drugs in the presence of serum, the combination of amphotericin B and flucytosine was not additive or synergistic against C. albicans. Concomitant use of amphotericin B and flucytosine may increase the toxicity of flucytosine, possibly by increasing cellular uptake and/or by decreasing renal excretion of the drug. If flucytosine is used in conjunction with amphotericin B, especially in HIV-infected patients, serum flucytosine concentrations and blood cell counts should be carefully monitored. In in vitro studies, the combination of flucytosine and fluconazole or itraconazole was synergistic, additive, or indifferent against Cryptococcus neoformans; there was no evidence of antagonism. The combination of fluconazole and flucytosine generally did not exert a synergistic effect against C. neoformans isolates that had fluconazole MICs of 8 ug/mL or greater. Synergism also has been demonstrated when the combination of fluconazole and flucytosine was evaluated in vivo in a murine model of cryptococcal meningitis. It has been suggested that synergism between the drugs may occur because fluconazole damages the fungal cell membrane allowing greater intracellular penetration of flucytosine. Cytarabine (cytosine arabinoside) reportedly antagonizes the antifungal activity of flucytosine, possibly by competitive inhibition. Concomitant use of the drugs is not recommended. Non-Human Toxicity Values LD50 Rat sc 3600 mg/kg LD50 Rat ip 3811 mg/kg LD50 Mouse iv 500 mg/kg LD50 Mouse sc 1000 mg/kg LD50 Mouse ip 1190 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antifungal Agents; Antimetabolites /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Flucytosine is included in the database. Flucytosine Capsules are indicated only in the treatment of serious infections caused by susceptible strains of Candida and/or Cryptococcus. Candida: Septicemia, endocarditis and urinary system infections have been effectively treated with flucytosine. Limited trials in pulmonary infections justify the use of flucytosine. Cryptococcus: Meningitis and pulmonary infections have been treated effectively. Studies in septicemias and urinary tract infections are limited, but good responses have been reported. /Included in US product label/ Flucytosine Capsules should be used in combination with amphotericin B for the treatment of systemic candidiasis and cryptococcosis because of the emergence of resistance to Flucytosine Capsules. /Included in US product label/ For more Therapeutic Uses (Complete) data for Flucytosine (10 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Use with extreme caution in patients with impaired renal function. Close monitoring of hematologic, renal and hepatic status of all patients is essential. These instructions should be thoroughly reviewed before administration of Flucytosine Capsules, USP. Flucytosine capsules must be given with extreme caution to patients with bone marrow depression. Patients may be more prone to depression of bone marrow function if they: 1) have a hematologic disease, 2) are being treated with radiation or drugs which depress bone marrow, or 3) have a history of treatment with such drugs or radiation. Bone marrow toxicity can be irreversible and may lead to death in immunosuppressed patients. Frequent monitoring of hepatic function and of the hematopoietic system is indicated during therapy. In addition to antiproliferative effects on the GI lining, adverse GI effects reported with flucytosine, which are sometimes severe, include anorexia, abdominal bloating, abdominal pain, diarrhea, dry mouth, duodenal ulcer, GI hemorrhage, nausea, vomiting, and ulcerative colitis. There are no adequate or controlled studies to date using flucytosine in pregnant women, and the drug should be used during pregnancy only when the potential benefits justify the possible risks to the fetus. For more Drug Warnings (Complete) data for Flucytosine (17 total), please visit the HSDB record page. Pharmacodynamics Flucytosine is an antimetabolite that acts as an antifungal agent with in vitro and in vivo activity against Candida and Cryptococcus. Flucytosine enters the fungal cell via cytosine permease; thus, flucytosine is metabolized to 5-fluorouracil within fungal organisms. The 5-fluorouracil is extensively incorporated into fungal RNA and inhibits synthesis of both DNA and RNA. The result is unbalanced growth and death of the fungal organism. Antifungal synergism between Ancobon and polyene antibiotics, particularly amphotericin B, has been reported. |
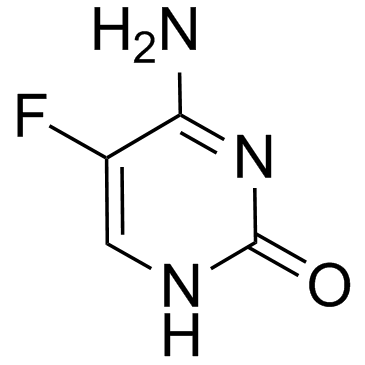
| 分子式 |
C4H4FN3O
|
|---|---|
| 分子量 |
129.0925
|
| 精确质量 |
129.033
|
| CAS号 |
2022-85-7
|
| 相关CAS号 |
Flucytosine-13C,15N2;1216616-31-7
|
| PubChem CID |
3366
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.7±0.1 g/cm3
|
| 沸点 |
298ºC
|
| 熔点 |
298-300 °C (dec.)(lit.)
|
| 蒸汽压 |
0.0492mmHg at 25°C
|
| 折射率 |
1.649
|
| LogP |
-2.36
|
| tPSA |
71.77
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
208
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
XRECTZIEBJDKEO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H4FN3O/c5-2-1-7-4(9)8-3(2)6/h1H,(H3,6,7,8,9)
|
| 化学名 |
6-amino-5-fluoro-1H-pyrimidin-2-one
|
| 别名 |
5-Fluorocytosine; NSC-103805; NSC103805; NSC 103805; Ro-2-9915; Ro2-9915; Ro 2-9915; Flucytosine, Flucytosin, Ancobon, Ancotil
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~16.67 mg/mL (~129.13 mM)
H2O : ~6.67 mg/mL (~51.67 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.67 mg/mL (12.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.67 mg/mL (12.94 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.67 mg/mL (12.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 配方 4 中的溶解度: 8.67 mg/mL (67.16 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.7465 mL | 38.7327 mL | 77.4653 mL | |
| 5 mM | 1.5493 mL | 7.7465 mL | 15.4931 mL | |
| 10 mM | 0.7747 mL | 3.8733 mL | 7.7465 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。