| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Adenylyl cyclase ( IC50 = 41 nM ); Adenylyl cyclase ( EC50 = 0.5 μM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:毛喉素可增加膜、细胞或组织制剂中的 cAMP 水平。 Forskolin 不仅激活 AC,还与某些其他蛋白质相互作用,包括葡萄糖转运蛋白和离子通道。 Forskolin 能够促进 AC 九种不同的跨膜亚型的激活,尽管对 AC9 的功效稍差,这可用于提供一种方法来识别和量化高亲和力结合位点,即 G 蛋白 (Gs) –AC复合体。由于 Forskolin 增强了 AC 活性,GPCR 的激活有助于 Forskolin 刺激细胞中 cAMP 的产生。毛喉素刺激腺苷酸环化酶活性,而不与细胞表面受体相互作用。毛喉素对 cAMP 的增强作用反过来又抑制嗜碱性粒细胞和肥大细胞脱颗粒和组胺释放,降低血压和眼压,抑制血小板聚集,促进血管舒张、支气管扩张和甲状腺激素分泌,并刺激脂肪细胞的脂肪分解。 Forskolin 抑制血小板激活因子 (PAF) 的结合,与 cAMP 形成无关,这可能是 Forskolin 直接作用于 PAF 或通过干扰 PAF 与受体位点结合的结果。毛喉素似乎还对几种膜转运蛋白有影响,并抑制红细胞、脂肪细胞、血小板和其他细胞中的葡萄糖转运。毛喉素用于治疗青光眼。细胞测定:4 天后,毛喉素浓度依赖性地降低人间充质干细胞增殖。此外,Forskolin 以剂量依赖性方式增加人间充质干细胞的碱性磷酸酶(ALP)表达。此外,Forskolin (10 μM) 显着刺激大鼠下丘脑-神经垂体 (H-NH) 系统释放加压素和催产素。
|
| 体内研究 (In Vivo) |
Forskolin(Fsk)处理的Mrp4-/-小鼠显示Ki67阳性和裂解的胱天蛋白酶3-阳性EC的数量增加,周细胞覆盖量显著减少,空袖的数量减少。在从P7到P12暴露于高氧(75%氧气)的幼崽中,Mrp4-/-小鼠的未血管化视网膜区域显著增加[3]。健康大鼠组的平均血糖为102.12±1.94 mg/dL,对照组为101.25±3.56,毛喉素组为103±2.08。数据显示,研究结束时,毛喉素组的血糖水平较低,根据应用的统计测试,差异显著(p=0.03)[6]。
|
| 酶活实验 |
体外激酶活性测定[5]
对于Jak3激酶测定,如上所述,使用Jak3抗体裂解、澄清和免疫沉淀Fsk处理的MT-2细胞。如前所述,在30°C下进行激酶反应20分钟。对于PKA激酶测定,裂解未处理的MT-2细胞,并如前所示将Jak3免疫沉淀并结合到PAS珠上。免疫沉淀的Jak3用激酶缓冲液(50 mm Hepes NaOH(pH 7.4)、10 mm MgCl2、0.5 mm EGTA、0.5 mm DTT、20μg/ml抑肽酶、10μg/ml亮蛋白肽、1μg/ml pepstatin A)洗涤,并与200μm ATP和纯化的蛋白激酶A催化亚基(PKAc)一起孵育,如图图例所示。激酶反应在32°C下进行30分钟,然后如前所述用冷激酶洗涤缓冲液剧烈洗涤珠粒。对于使用重组Jak3的[γ-32P]ATP放射性标记的激酶测定,根据制造商的说明,使用Lipofectamine 2000用野生型(WT)Jak3或激酶死亡的Jak3 K855A转染Hek293细胞。裂解细胞并用Jak3抗体免疫沉淀(如上所述)。在冷裂解缓冲液(如上所述)中洗涤Jak3结合的PAS珠三次,然后洗涤激酶缓冲液。通过向Jak3结合的PAS珠反应混合物中加入10μCi[γ-32P]ATP、10μm未标记的ATP和1μg纯化的PKAc来启动激酶反应。激酶反应在32°C下进行30分钟。将Jak3结合的PAS珠在放射免疫分析缓冲液(10 mm Tris-HCl,pH 7.4,75 mm NaCl,20 mm EDTA,10 mm EGTA,20 mm Na4P2O7,50 mm NaF,20 mm 2-甘油磷酸,1 mm对硝基苯磷酸,0.1%Triton X-100)中洗涤三次,并在激酶洗涤缓冲液(如上所述)中洗涤一次。通过加入2×SDS-PAGE样品缓冲液,然后加入SDS-PAGE来停止反应。从PVDF膜上切下考马斯染色的Jak3带,并进行磷酸氨基酸分析。 |
| 细胞实验 |
在 96 孔板中,将 5×104 MT-2 或 Quiescent Kit 225 细胞接种到每个孔中。然后,用浓度为 1、5、10、25、50 和 100 μM 的毛喉素或 1% DMSO(载体)预处理细胞一小时。在 37°C 和 IL-2 刺激下培养 20 小时后,收获细胞。对照细胞用 1% DMSO 处理 20 小时。在孵育的最后 4 小时内,[3H]胸苷以 0.5 μCi/200 μL 的浓度脉冲进入细胞。使用液体闪烁计数,将细胞收集到玻璃纤维过滤器上进行分析。
|
| 动物实验 |
Mice: Mice C57BL/6J are employed. established and frequently backcrossed Mrp4-knockout miceto the C57BL/6J mice At postnatal days 4 (P4) and 5, neonatal mice receive an intraperitoneal injection of forskolin (P5). The controls are mice that have been injected with DMSO. After the P6 euthanasia of the treated mice, their retinas are separated for whole-mount immunohistochemistry (IHC). To compare the retinal vascular phenotypes of WT and Mrp4-deficient mice, the optimal concentration of Forskolin was found to be 1.0 μg/50 μL (0.3 mg/kg) at P4 and 1.5 μg/50 μL (0.5 mg/kg) at P5. This was achieved by testing the effects of different Forskolin concentrations on the survival rate and retinal vasculature.
Rats: Four groups of male Wistar rats, ages 10–14 weeks, with mean weights of 300 g±50 g, are created: eight are kept in good health, and 19 are experimentally made to develop diabetes. For eight weeks, either 6 mg/kg of forskolin per day is given orally to both diabetic and healthy rats as a control. Before and after Forskolin treatment, each group's blood glucose levels are measured. After eight weeks of the prescribed treatment and two weeks following the confirmation of diabetes (three weeks following the induction), the diabetic rats are tested. Experimental approach: Male Sprague-Dawley rats were treated with either CCl4 and/or forskolin for 6 consecutive weeks. Serum hepatotoxicity markers were determined, and histopathological evaluation was performed. Hepatic fibrosis was assessed by measuring α-SMA expression and collagen deposition by Masson's trichrome staining and hydroxyproline content. The effects of forskolin on oxidative stress markers (GSH, GPx, lipid peroxides), inflammatory markers (NF-κB, TNF-α, COX-2, IL-1β), TGF-β1 and Hh signalling markers (Ptch-1, Smo, Gli-2) were also assessed. Key results: Hepatic fibrosis induced by CCl4 was significantly reduced by forskolin, as indicated by decreased α-SMA expression and collagen deposition. Forskolin co-treatment significantly attenuated oxidative stress and inflammation, reduced TGF-β1 levels and down-regulated mRNA expression of Ptch-1, Smo and Gli-2 through cAMP-dependent PKA activation. [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
rat LD50 oral 2550 mg/kg Medicinal Research Reviews., 3(201), 1983 [PMID:6345959]
rat LD50 intraperitoneal 92 mg/kg Journal of Medicinal Chemistry., 31(1872), 1988 mouse LD50 oral 3100 mg/kg Medicinal Research Reviews., 3(201), 1983 [PMID:6345959] mouse LD50 intraperitoneal 68 mg/kg BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) Journal of Ethnopharmacology., 3(1), 1981 [PMID:7193263] |
| 参考文献 | |
| 其他信息 |
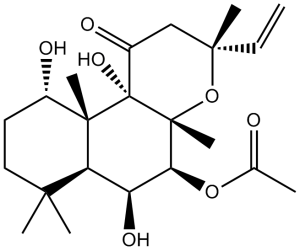
Forskolin is a labdane diterpenoid isolated from the Indian Coleus plant. It has a role as a plant metabolite, an anti-HIV agent, a protein kinase A agonist, an adenylate cyclase agonist, an antihypertensive agent and a platelet aggregation inhibitor. It is a labdane diterpenoid, an acetate ester, an organic heterotricyclic compound, a triol, a cyclic ketone and a tertiary alpha-hydroxy ketone.
Potent activator of the adenylate cyclase system and the biosynthesis of cyclic AMP. From the plant Coleus forskohlii. Has antihypertensive, positive inotropic, platelet aggregation inhibitory, and smooth muscle relaxant activities; also lowers intraocular pressure and promotes release of hormones from the pituitary gland. Forskolin has been reported in Plectranthus, Apis cerana, and Plectranthus barbatus with data available. Potent activator of the adenylate cyclase system and the biosynthesis of cyclic AMP. From the plant Coleus forskohlii. Has antihypertensive, positive ionotropic, platelet aggregation inhibitory, and smooth muscle relaxant activities; also lowers intraocular pressure and promotes release of hormones from the pituitary gland. Potent activator of the adenylate cyclase system and the biosynthesis of cyclic AMP. From the plant Coleus forskohlii. Has antihypertensive, positive inotropic, platelet aggregation inhibitory, and smooth muscle relaxant activities; also lowers intraocular pressure and promotes release of hormones from the pituitary gland. 1. As initially shown by Seamon and Daly, the diterpene forskolin directly activates adenylyl cyclase (AC) and raises cyclic AMP levels in a wide variety of cell types. In this review, we discuss several aspects of forskolin action that are often unappreciated. These include the utility of labeled forskolin as a means to quantitate the number of AC molecules; results of those types of studies, coupled with efforts to increase AC expression, document that such expression stoichiometrically limits cyclic AMP formation by hormones and neurotransmitters. 2. Response to forskolin is also strongly influenced by the activation of AC by the heterotrimeric G-protein, Gs. Gs-promoted enhancement of AC activity in response to forskolin occurs not only when cells are incubated with exogenously administered agonists that activate G-protein-coupled receptors but also by agonists that can be endogenously released by cells. 3. Such agonists, which include ATP and prostaglandins, serve as autocrine/paracrine regulators of cellular levels of cyclic AMP under "basal" conditions and also in response to forskolin and to agonists that promote release of such regulators. 4. The ability of forskolin to prominently activate cyclic AMP generation has proved valuable for understanding stoichiometry of the multiple components involved in "basal" cyclic AMP formation, in enzymologic studies of AC as well as in defining responses to cyclic AMP in cells within and outside the nervous system.[1] Background and purpose: Liver fibrosis is one of the leading causes of morbidity and mortality worldwide with very limited therapeutic options. Given the pivotal role of activated hepatic stellate cells in liver fibrosis, attention has been directed towards the signalling pathways underlying their activation and fibrogenic functions. Recently, the hedgehog (Hh) signalling pathway has been identified as a potentially important therapeutic target in liver fibrosis. The present study was designed to explore the antifibrotic effects of the potent Hh signalling inhibitor, forskolin, and the possible molecular mechanisms underlying these effects. Conclusion and implications: In our model, forskolin exerted promising antifibrotic effects which could be partly attributed to its antioxidant and anti-inflammatory effects, as well as to its inhibition of Hh signalling, mediated by cAMP-dependent activation of PKA.[4] Cytokine-mediated regulation of T-cell activity involves a complex interplay between key signal transduction pathways. Determining how these signaling pathways cross-talk is essential to understanding T-cell function and dysfunction. In this work, we provide evidence that cross-talk exists between at least two signaling pathways: the Jak3/Stat5 and cAMP-mediated cascades. The adenylate cyclase activator forskolin (Fsk) significantly increased intracellular cAMP levels and reduced proliferation of the human T-cells via inhibition of cell cycle regulatory genes but did not induce apoptosis. To determine this inhibitory mechanism, effects of Fsk on IL-2 signaling was investigated. Fsk treatment of MT-2 and Kit 225 T-cells inhibited IL-2-induced Stat5a/b tyrosine and serine phosphorylation, nuclear translocation, and DNA binding activity. Fsk treatment also uncoupled IL-2 induced association of the IL-2Rβ and γc chain, consequently blocking Jak3 activation. Interestingly, phosphoamino acid analysis revealed that Fsk-treated cells resulted in elevated serine phosphorylation of Jak3 but not Stat5, suggesting that Fsk can negatively regulate Jak3 activity possibly mediated through PKA. Indeed, in vitro kinase assays and small molecule inhibition studies indicated that PKA can directly serine phosphorylate and functionally inactivate Jak3. Taken together, these findings suggest that Fsk activation of adenylate cyclase and PKA can negatively regulate IL-2 signaling at multiple levels that include IL-2R complex formation and Jak3/Stat5 activation.[5] Forskolin is a diterpene derived from the plant Coleus forskohlii. Forskolin activates adenylate cyclase, which increases intracellular cAMP levels. The antioxidant and antiinflammatory action of forskolin is due to inhibition of macrophage activation with a subsequent reduction in thromboxane B2 and superoxide levels. These characteristics have made forskolin an effective medication for heart disease, hypertension, diabetes, and asthma. Here, we evaluated the effects of chronic forskolin administration on blood glucose and oxidative stress in 19 male Wistar rats with streptozotocin-induced diabetes compared to 8 healthy male Wistar rats. Rats were treated with forskolin, delivered daily for 8 weeks. Glucose was assessed by measuring fasting blood glucose in diabetic rats and with an oral glucose tolerance test (OGTT) in healthy rats. Oxidative stress was assessed by measuring 8-hydroxydeoxyguanosine (8‑OHdG) in 24-h urine samples. In diabetic rats, without forskolin, fasting blood glucose was significantly higher at the end than at the beginning of the experiment (8 weeks). In both healthy and diabetic rats, forskolin treatment lowered the fasting glucose at the end of the experiment but no effect was found on oral glucose tolerance. The 8-OHdG levels tended to be less elevated in forskolin-treated than in untreated group. Our results showed that chronic administration of forskolin decreased fasting blood glucose levels; however, the reductions of 8-OHdG were not statistically significant.[6] |
| 分子式 |
C22H34O7
|
|
|---|---|---|
| 分子量 |
410.5
|
|
| 精确质量 |
410.23
|
|
| 元素分析 |
C, 64.37; H, 8.35; O, 27.28
|
|
| CAS号 |
66575-29-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
47936
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
519.9±50.0 °C at 760 mmHg
|
|
| 熔点 |
282-232ºC
|
|
| 闪点 |
171.8±23.6 °C
|
|
| 蒸汽压 |
0.0±3.1 mmHg at 25°C
|
|
| 折射率 |
1.552
|
|
| LogP |
3.4
|
|
| tPSA |
113.29
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
747
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
O1[C@](C([H])=C([H])[H])(C([H])([H])[H])C([H])([H])C([C@]2([C@@]1(C([H])([H])[H])[C@]([H])([C@]([H])([C@@]1([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])C([H])([H])[C@@]([H])([C@@]12C([H])([H])[H])O[H])O[H])OC(C([H])([H])[H])=O)O[H])=O
|
|
| InChi Key |
OHCQJHSOBUTRHG-KGGHGJDLSA-N
|
|
| InChi Code |
InChI=1S/C22H34O7/c1-8-19(5)11-14(25)22(27)20(6)13(24)9-10-18(3,4)16(20)15(26)17(28-12(2)23)21(22,7)29-19/h8,13,15-17,24,26-27H,1,9-11H2,2-7H3/t13-,15-,16-,17-,19-,20-,21+,22-/m0/s1
|
|
| 化学名 |
[(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.09 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4361 mL | 12.1803 mL | 24.3605 mL | |
| 5 mM | 0.4872 mL | 2.4361 mL | 4.8721 mL | |
| 10 mM | 0.2436 mL | 1.2180 mL | 2.4361 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01254006 | Completed | Drug: forskolin, rutin and vitamins B1 and B2 |
Glaucoma | University of Roma La Sapienza | N/A | Not Applicable |
|
|
|