| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg | |||
| Other Sizes |
| 靶点 |
Endogenous metabolite; glycine-conjugated secondary bile acid
|
|---|---|
| 体外研究 (In Vitro) |
石胆酸(LCA)是一种与胆汁淤积等不良反应相关的胆汁酸,在体内主要以Glycolithocholic acid(GLCA)和tauro-LCA(TLCA)的结合物存在。三苯氧胺与胆汁淤积的发展有关,它抑制磺基转移酶2A1(SULT2A1)催化的脱氢表雄酮(DHEA)磺化。本研究旨在表征LCA、GLCA和TLCA的磺化作用,并研究选择性雌激素受体调节剂(SERM)类的三苯乙烯(氯米芬、三苯氧胺、托瑞米芬、奥赛美芬、屈洛昔芬)、苯并噻吩(雷洛昔芬、阿唑昔芬)、四氢萘(拉索昔芬、萘呋辛)、吲哚(巴多昔芬)和苯并吡喃(acolbifene)是否抑制LCA、GLPA和TLCA磺化。人重组SULT2A1,但不是SULT2B1b或SULT1E1,催化LCA、GLCA和TLCA磺化,而这些酶中的每一种都催化DHEA磺化。LCA、GLCA和TLCA磺化由人肝细胞溶质催化,SULT2A1遵循底物抑制模型,具有相当的表观Km值(≤1µm)。每种SERM都以不同的效力和酶抑制模式抑制LCA、GLCA和TLCA磺化。通过对托瑞米芬、巴多昔芬和拉索菲芬进行结构修饰,LCA磺化的效力和抑制程度减弱或增加。在HepG2人肝细胞癌细胞中也观察到雷洛昔芬、巴多昔芬和阿可联苯对LCA磺化的抑制作用。总体而言,在所研究的SERM中,巴多昔芬和雷洛昔芬是LCA、GLCA和TLCA磺化最有效的抑制剂。这些发现为特定SERM的结构特征提供了见解,这些特征有助于抑制SULT2A1催化的LCA磺化。SERM对LCA、GLCA和TLCA解毒的抑制可能为SERM相关的不良反应提供生化基础[3]。
|
| 体内研究 (In Vivo) |
在杂合FH IIa型患者中,我们观察到与对照组的相应值相比,甘鹅脱氧胆酸、甘熊脱氧胆酸和Glycolithocholic acid的摩尔百分比显著降低,牛磺脱氧胆酸显著增加。在普罗布考治疗16周后,对6名患者的胆汁分析进行了复查。普罗布考显著降低了血清胆固醇水平。然而,治疗没有改变胆汁脂质组成和单个胆汁酸比例。结果表明,大多数杂合性FH患者胆汁过饱和,易形成胆固醇结石。此外,普罗布考降低血清胆固醇的机制似乎与胆汁脂质代谢的任何变化无关。[1]
结果:本研究纳入了32名UC患者和23名HC患者。研究发现,与HC相比,UC患者的肠道微生物群多样性降低。UC患者的厚壁菌门、梭菌IV、丁球菌、梭菌XlVa、粪杆菌和Roseburia显著降低(分别为P=0.75E-05、P=0.28E-07、P=0.0002、P=0.003、P=0.0003和P=0.0004)。UC组中变形杆菌、大肠杆菌、肠球菌、克雷伯氏菌和链球菌显著富集(分别为P=2.99E-09、P=3.63E-05、P=8.59E-05、P=0.003和P=0.016)。UC患者粪便中的继发性BAs,如石胆酸、脱氧胆酸、糖脱氧胆酸、Glycolithocholic acid和牛磺胆酸的浓度显著低于HCs(分别为P=8.1E-08、P=1.2E-07、P=3.5E-04、P=1.9E-03和P=1.8E-02),并与丁球菌、玫瑰孢菌、梭菌IV、粪杆菌和梭菌XlVb呈正相关(P<0.01)。UC患者的原发性BA,如牛磺胆酸、胆酸、牛磺脱氧胆酸和甘鹅脱氧胆酸的浓度显著高于HC患者(分别为P=5.3E-03、P=4E-02、P=0.042和P=0.045),并且与肠球菌、克雷伯氏菌、链球菌、乳杆菌和促炎细胞因子呈正相关(P<0.01)。UC患者TGR5的表达显著升高(0.019±0.013 vs 0.006±0.003,P=0.0003)。UC患者结肠黏膜标本中VDR表达显著降低(0.011±0.007 vs 0.016±0.004,P=0.033)。 结论:粪便BA谱与肠道微生物群和血清炎性细胞因子密切相关。肠道微生物群失调和粪便BAs结构改变可能通过BA受体TGR5和VDR参与调节炎症反应[2]。 |
| 酶活实验 |
LCA、GLCA/Glycolithocholic acid、TLCA和DHEA磺化试验的优化[3]
之前已经优化了人肝细胞质中的LCA磺化试验(Bansal和Lau,2016a)。LCA和GLCA/Glycolithocholic acid磺化在100µg细胞质蛋白时线性增加,而TLCA磺化在80µg细胞质蛋白质时线性增加(补充图S1,A-C)。重组SULT2A1催化的LCA、Glycolithocholic acid/GLCA和TLCA磺化在5µg酶下呈线性(补充图S1,D-F)。人肝细胞溶质催化LCA磺化反应在45分钟内呈线性,而GLCA在45分钟后呈线性。。。 |
| 动物实验 |
Background: Gut microbiota and its metabolites may be involved in the pathogenesis of inflammatory bowel disease. Several clinical studies have recently shown that patients with ulcerative colitis (UC) have altered profiles of fecal bile acids (BAs). It was observed that BA receptors Takeda G-protein-coupled receptor 5 (TGR5) and vitamin D receptor (VDR) participate in intestinal inflammatory responses by regulating NF-ĸB signaling. We hypothesized that altered profiles of fecal BAs might be correlated with gut microbiota and inflammatory responses in patients with UC.
Aim: To investigate the changes in fecal BAs and analyze the relationship of BAs with gut microbiota and inflammation in patients with UC.
Methods: The present study used 16S rDNA sequencing technology to detect the differences in the intestinal flora between UC patients and healthy controls (HCs). Fecal BAs were measured by targeted metabolomics approaches. Mucosal TGR5 and VDR expression was analyzed using immunohistochemistry, and serum inflammatory cytokine levels were detected by ELISA.[2]
|
| 参考文献 |
|
| 其他信息 |
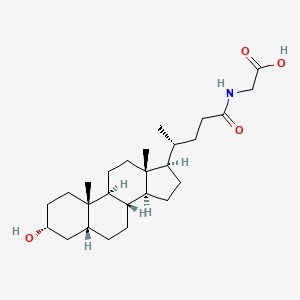
Glycolithocholic acid is the glycine conjugate of lithocholic acid. It is a bile acid glycine conjugate and a N-acylglycine. It is functionally related to a lithocholic acid. It is a conjugate acid of a glycolithocholate.
Glycolithocholic acid has been reported in Homo sapiens and Bos taurus with data available. See also: Glycolithocholate (annotation moved to). Background & aims: Higher serum bile acid levels are associated with an increased risk of cirrhosis and liver-related morbidity and mortality. Herein, we report secondary analyses of aldafermin, an engineered analogue of the gut hormone fibroblast growth factor 19, on the circulating bile acid profile in prospective, phase II studies in patients with metabolic or cholestatic liver disease. Methods: One hundred and seventy-six patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH) and fibrosis and elevated liver fat content (≥8% by magnetic resonance imaging-proton density fat fraction) received 0.3 mg (n = 23), 1 mg (n = 49), 3 mg (n = 49), 6 mg (n = 28) aldafermin or placebo (n = 27) for 12 weeks. Sixty-two patients with primary sclerosing cholangitis (PSC) and elevated alkaline phosphatase (>1.5× upper limit of normal) received 1 mg (n = 21), 3 mg (n = 21) aldafermin or placebo (n = 20) for 12 weeks. Serum samples were collected on day 1 and week 12 for determination of bile acid profile and neoepitope-specific N-terminal pro-peptide of type III collagen (Pro-C3), a direct measure of fibrogenesis. Results: Treatment with aldafermin resulted in significant dose-dependent reductions in serum bile acids. In particular, bile acids with higher hydrophobicity indices, such as deoxycholic acid, lithocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, and glycocholic acid, were markedly lowered by aldafermin in both NASH and PSC populations. Moreover, aldafermin predominantly suppressed the glycine-conjugated bile acids, rather than the taurine-conjugated bile acids. Changes in levels of bile acids correlated with changes in the novel fibrogenesis marker Pro-C3, which detects a neo-epitope of the type III collagen during its formation, in the pooled NASH and PSC populations. Conclusions: Aldafermin markedly reduced major hydrophobic bile acids that have greater detergent activity and cytotoxicity. Our data provide evidence that bile acids may contribute to sustaining a pro-fibrogenic microenvironment in the liver across metabolic and cholestatic liver diseases. Lay summary: Aldafermin is an analogue of a gut hormone, which is in development as a treatment for patients with chronic liver disease. Herein, we show that aldafermin can potently and robustly suppress the toxic, hydrophobic bile acids irrespective of disease aetiology. The therapeutic strategy utilising aldafermin may be broadly applicable to other chronic gastrointestinal and liver disorders. Clinical trials registration: The study is registered at Clinicaltrials.govNCT02443116 and NCT02704364.[4] |
| 分子式 |
C26H43NO4
|
|---|---|
| 分子量 |
433.63
|
| 精确质量 |
433.319
|
| 元素分析 |
C, 72.02; H, 10.00; N, 3.23; O, 14.76
|
| CAS号 |
474-74-8
|
| 相关CAS号 |
24404-83-9 (mono-hydrochloride salt)
|
| PubChem CID |
115245
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.112g/cm3
|
| 沸点 |
619.7ºC at 760mmHg
|
| 熔点 |
212-214°C (lit.)
|
| 闪点 |
328.6ºC
|
| LogP |
5.014
|
| tPSA |
86.63
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
695
|
| 定义原子立体中心数目 |
9
|
| SMILES |
C[C@H](CCC(=O)NCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@H]4[C@@]3(CC[C@H](C4)O)C)C
|
| InChi Key |
XBSQTYHEGZTYJE-OETIFKLTSA-N
|
| InChi Code |
InChI=1S/C26H43NO4/c1-16(4-9-23(29)27-15-24(30)31)20-7-8-21-19-6-5-17-14-18(28)10-12-25(17,2)22(19)11-13-26(20,21)3/h16-22,28H,4-15H2,1-3H3,(H,27,29)(H,30,31)/t16-,17-,18-,19+,20-,21+,22+,25+,26-/m1/s1
|
| 化学名 |
2-[[(4R)-4-[(3R,5R,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]acetic acid
|
| 别名 |
Lithocholic acid glycine conjugate; Lithocholylglycine; Glycolithocholic acid; Lithocholylglycine; 474-74-8; Lithocholic acid glycine conjugate; Glycine, N-[(3a,5b)-3-hydroxy-24-oxocholan-24-yl]-; Q53GV75CJG; CHEMBL258818; CHEBI:37998; Glycolithocholic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~115.31 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (2.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3061 mL | 11.5306 mL | 23.0611 mL | |
| 5 mM | 0.4612 mL | 2.3061 mL | 4.6122 mL | |
| 10 mM | 0.2306 mL | 1.1531 mL | 2.3061 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。