| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
NF-κB; TNF-α; IKKβ; IL-6
|
|---|---|
| 体外研究 (In Vitro) |
Homoplantaginin 还原 DPPH 自由基的 IC50 为 0.35 μg/mL。Homoplantaginin(0.1-100μg/mL)除了增加暴露于 H2O2 的人肝细胞 HL-7702 细胞上清液中的谷胱甘肽、谷胱甘肽过氧化物酶和超氧化物歧化酶外,还显着减少乳酸脱氢酶的渗漏[1]。棕榈酸 (100 μM) 诱导的 Toll 样受体 4 表达会被Homoplantaginin (0.1、1、10 μM) 剂量依赖性地降低。通过抑制 NLRP3 和 caspase-1 蛋白(活性氧敏感的硫氧还蛋白相互作用蛋白),Homoplantaginin严格调节棕榈酸诱导的活性氧,以防止 NLRP3 炎症小体激活 [2]。 Homoplantaginin 预处理显着降低棕榈酸诱导的人脐静脉内皮细胞中 TNF-α 和 IL-6 的 mRNA 表达以及 IKKβ 和 NF-κB p65 磷酸化。 Homoplantaginin 显着改变 IRS-1 的 Ser/Thr 磷酸化,增强 Akt 和内皮一氧化氮合酶磷酸化,并在胰岛素存在下增加 NO 产生 [3]。
Homoplantaginin [1] 的抗氧化作用 为了评价Homoplantaginin对自由基的直接清除作用,我们测定了一种稳定的自由基DPPH的清除活性。如图2所示,当浓度达到1.6 μg/ml时,Homoplantaginin降低DPPH自由基水平,显示出清除自由基的作用,IC50值为0.35 μg/ml。l-抗坏血酸具有清除自由基的作用,IC50值为0.43 μg/ml。 细胞活力暴露于Homoplantaginin体外[1] 将HL-7702细胞分别暴露于Homoplantaginin(0.1、1、10、50、100、200 μg/ml)中72 h,通过MTT法测定活细胞数,评估细胞生长抑制率。如图3所示,这些浓度的Homoplantaginin均未引起任何明显的细胞毒性(与未处理的细胞相比P < 0.05)。 LDH释放抑制[1] 采用H2O2暴露HL-7702细胞模型,研究Homoplantaginin对肝细胞损伤的保护作用。暴露于H2O2中18小时显著增加LDH向介质的泄漏。如图4所示,对照细胞上清液中LDH水平为21 U/ml, H2O2胁迫下LDH水平升高至122 U/ml。Homoplantaginin预处理HL-7702细胞可显著抑制LDH渗漏,且呈剂量依赖性。在浓度为0.1、1、10、50和100 μg/ml时,LDH水平分别降至73.5±4.4、65.2±5.1、58.4±3.2、52.1±2.6和41.6±4.3 U/ml(图4)。 GSH、GSH- px、SOD升高[1] 测定h2o2损伤HL-7702细胞中GSH、GSH- px和SOD的活性。结果见表1。h2o2损伤细胞的GSH水平从58.4±4.5 nmol/mg蛋白的正常值降至40.5±5.7 nmol/mg蛋白。Homoplantaginin阻止了H2O2对GSH的消耗。预处理24 h后,在0.1 ~ 100 μg/ml浓度范围内,GSH呈剂量依赖性升高(表1)。 然后我们检测了Homoplantaginin对肝细胞GSH-Px和SOD的影响。HL-7702细胞暴露于H2O2后,GSH-Px和SOD活性降低(表1)。Homoplantaginin增加两种抗氧化酶的活性呈剂量依赖性(表1)。 棕榈酸(PA)诱导的血管内皮炎症在血管疾病的发生发展中起着至关重要的作用。本文研究了中药鼠尾草中主要类黄酮Homoplantaginin的作用。研究了pa处理的人脐静脉内皮细胞炎症及其分子机制。首先,我们发现Homoplantaginin(0.1,1,10 μM)剂量依赖性地降低了PA (100 μM)诱导的toll样受体-4的表达。在脂多糖刺激下,进一步证实了Homoplantaginin的抑制作用。此外,在PA处理下,下游适应蛋白包括髓样分化初级反应基因88、toll/白细胞介素-1受体结构域含有适配器诱导干扰素-β和肿瘤坏死因子受体相关因子-6被Homoplantaginin成功抑制。此外,我们发现Homoplantaginin通过抑制活性氧物种敏感的硫氧还蛋白相互作用蛋白NLRP3和caspase-1,严格控制pa诱导的活性氧来阻止核苷酸结合结构域样受体3 (NLRP3)炎性体的激活。同时,也降低了炎症介质(白细胞介素-1β、细胞间黏附分子-1和单核细胞趋化蛋白-1)的蛋白和mRNA水平。此外,Homoplantaginin还能恢复pa损伤的一氧化氮生成。综上所述,这些结果表明,Homoplantaginin通过抑制toll样受体-4和NLRP3通路,保护内皮细胞免受pa诱导的内皮炎症的改善,并恢复一氧化氮的产生,这表明它可能是一个潜在的候选物,可以进一步开发用于预防和治疗血管疾病。[2] 最近的数据表明,炎症在胰岛素抵抗的发展中起着重要作用。本研究旨在研究中药鼠尾草中的类黄酮Homoplantaginin的活性。内皮细胞中棕榈酸(PA)诱导的胰岛素敏感性及其抗炎特性的潜在机制。前处理的Homoplantaginin对人脐静脉内皮细胞(HUVECs)有显著抑制PA诱导的肿瘤坏死因子-α (TNF-α)和白细胞介素-6 (IL-6) mRNA表达,抑制κB激酶β (IKKβ)和核因子-κB (NF-κB) p65磷酸化。对于PA损伤胰岛素受体底物-1 (IRS-1)的胰岛素依赖性酪氨酸磷酸化和一氧化氮(NO)的产生,前处理的Homoplantaginin可以有效逆转PA的作用。此外,Homoplantaginin显著调节IRS-1的丝氨酸/苏氨酸磷酸化,改善Akt和内皮型一氧化氮合酶(eNOS)的磷酸化,并在胰岛素存在下增加NO的产生。综上所述,我们的研究结果表明,Homoplantaginin通过抑制炎症和通过IKKβ/IRS-1/pAkt/peNOS通路调节细胞信号传导来改善内皮胰岛素抵抗,这表明它可能用于预防和治疗与胰岛素抵抗相关的内皮功能障碍。[3] |
| 体内研究 (In Vivo) |
Homoplantaginin (25-100mg/kg) 显着降低血清丙氨酸和天冬氨酸转氨酶水平的升高,并降低 TNF-α 和 IL-1 水平。相同的过程还增加了肝匀浆中 GSH、GSH-Px 和 SOD 的水平,并降低了硫代巴比妥酸反应物质的含量[1]。Homoplantaginin吸收迅速(Tmax=16.00±8.94min),其平均Cmax范围在0.77至1.27 nmol/mL之间。据估计,只有 0.75% 的口服生物利用度是绝对的。
Homoplantaginin对BCG/LPS诱导的ILI的治疗作用 采用卡介苗/LPS诱导小鼠ILI的方法,评价了Homoplantaginin的治疗作用。在该模型中,小鼠表现出严重的肝损伤,血清转氨酶(ALT、AST)升高,促炎细胞因子如TNF-α、IL-1升高,多种抗氧化酶如GSH、GSH- px、SOD降低(表2、表3)。Homoplantaginin明显抑制ILI的发生。如表2所示,给药10 d后,小鼠血清中谷丙转氨酶和谷丙转氨酶水平显著降低。ALT和AST的降低呈剂量依赖性(25、50、100 mg/kg/d)。另一方面,在连续给药期间没有观察到明显的体重减轻(数据未显示)。同样,与BCG + lps处理的小鼠相比,联苯酯(100 mg/kg)降低了ALT和AST的水平(表2)。 抗氧化酶升高[1] 测定小鼠肝组织中GSH、GSH- px、SOD水平。如表3所示,Homoplantaginin(25、50、100 mg/kg)显著提高GSH、GSH- px活性(P < 0.01),微弱提高SOD活性(25、50 mg/kg, P < 0.05)。100 mg/kg,与未处理模型相比P < 0.05,表3)。 TBARS下降[1] 小鼠BCG启动后注射LPS导致肝组织TBARS水平显著升高(表3)。Homoplantaginin显著降低TBARS升高(25 mg/kg, P > 0.05);50和100 mg/kg,与未处理模型相比P < 0.05,表3)。 TNF-α和IL-1 [1] 如表4所示,BCG/ lps诱导小鼠血清中TNF-α和IL-1水平明显高于对照组。Homoplantaginin可明显抵消ILI小鼠血清中TNF-α和IL-1水平升高(25 mg/kg, P < 0.05);50和100 mg/kg,与未处理模型相比P < 0.01,表4)。 Homoplantaginin对肝脏组织学的影响[1] 正常小鼠肝脏组织病理学检查未见组织学异常(图5A)。LPS注射bcg小鼠,可见肝窦充血,炎性细胞散在浸润,出现点状坏死、碎片状坏死、桥状坏死,炎性细胞在坏死组织周围排列(图5B)。在Homoplantaginin(25、50、100 mg/kg/d)处理的小鼠中,坏死的面积和程度减少,炎症细胞的迁移减少(图5D-F)。表2显示了各组小鼠的肝损伤类别。Homoplantaginin治疗组(50、100 mg/kg/d, P < 0.05)大鼠肝损伤程度明显改善(表2)。 本研究旨在研究鼠尾草主要活性成分 的药动学特性。本研究测定了Homoplantaginin的有效分配系数、大鼠肠段的原位吸收以及大鼠肠道细菌对Homoplantaginin的体外生物转化。此外,大鼠还分别通过静脉、腹腔和口服给药给药Homoplantaginin。采用高效液相色谱法测定Homoplantaginin和Homoplantaginin代谢物hispidulin的浓度。经静脉、腹膜注射后,无法测定海鞘磷脂的浓度。口服后同时定量hispidulin和homintaginin, homintaginin吸收迅速(Tmax=16.00±8.94min),平均Cmax在0.77 ~ 1.27nmol/mL之间。计算出其绝对口服生物利用度仅为0.75%,曲线下面积(AUC)约为Homoplantaginin的5.4倍。大鼠肠道细菌对Homoplantaginin的生物转化可能是其口服生物利用度较差的原因。[4] |
| 酶活实验 |
抗氧化活性[1]
Homoplantaginin的抗氧化活性通过dpph清除试验进行了评估(Watjen et al., 2007, Yang et al., 2005)。将含Homoplantaginin(实验化合物)和40 μl DPPH(1,1-二苯基-2-picrylhydrazyl自由基溶解于MeOH)的反应混合物160微升置于96孔板中,暗孵育30 min。对照组用蒸馏水代替实验化合物溶液。反应后,用517 nm比色法测定剩余的DPPH。从反应混合物的吸光度中减去Homoplantaginin的吸光度作为空白。自由基清除活性计算为100 × (ODcontrol−ODtest)/ODcontrol。IC50值定义为抑制DPPH自由基形成50%所需的测试化合物浓度。l-抗坏血酸,一种众所周知的抗氧化剂,被用作阳性对照(Yang et al., 2005)。 Homoplantaginin在体外的生物转化[4] 采用大鼠肠道菌群和含2.16 μMHomoplantaginin的GAM溶液(1:9,v:v)组成完整的培养体系进行肠道细菌培养。然后将混合物在37℃厌氧培养中孵育。在设计时间(5、10、20、30、45、60、90、120、180、300、480、720 min),取出反应混合物,用甲醇(1:1,v:v)萃取。旋流3min, 12000 rpm离心10min,最后取20 μL上清液入HPLC系统分析。条件与2.3相同。 |
| 细胞实验 |
MTT 测定用于评估培养细胞的活力。Homoplantaginin以不同浓度(0.1、1、3、10、30、100 M)作用于人脐静脉内皮细胞 48 小时。然后,每孔加入 20 μL MTT (5 mg/mL),在 37°C 下再培养 4 小时。除去上清液后,添加 DMSO 以帮助溶解甲臜晶体。在 540 nm[3] 处测量吸光度。
细胞培养和活力[1] 人肝细胞系HL-7702在rmi -1640中维持,rmi -1640中添加10% (v/v)热灭活胎牛血清、青霉素-链霉素(100 IU/ml - 100 μg/ml)、2 mM谷氨酰胺和10 mM Hepes缓冲液,在37℃的潮湿环境中(5% CO2, 95%空气)。采用3-[4,5-二甲基噻唑-2-基]-2,5-二苯基溴化四氮唑(MTT)测定法评估细胞生长和活力,详见其他文献(Zhang et al., 2008a)。 H2O2诱导的氧化应激[1] HL-7702细胞以3 × 105 / ml的密度在6孔板中培养,并使其生长到所需的合流度。用不同浓度(0.1 ~ 100 μg/ml)的Homoplantaginin预处理细胞24 h,然后暴露于750 μM的H2O2中18 h (Wang et al., 2005a)。未经H2O2处理的细胞在与实验方案中使用的H2O2相同的条件下孵育。 细胞内ROS水平测定[2] 将HUVECs (5 × 104细胞/孔)接种于6孔板中,在5% CO2培养箱中37℃保存48小时。然后将培养基替换为无血清培养基,使细胞饥饿2.5小时。细胞分别用PA (100 μM)处理1.5、3和6小时,或在PA (100 μM)处理3小时之前,用不同浓度的Homoplantaginin(0.1、1和10 μM)或Sal (500 μM)预处理0.5小时。收获细胞,用10 μM DCFH-DA在37℃下暗室孵育20分钟。用冷磷酸盐缓冲盐水洗涤两次后,用FACS Calibur流式细胞仪 在激发波长为488 nm,发射波长为525 nm.24下对细胞进行分析利用Cell Quest软件计算二氯荧光素(DCF)的荧光分布。 Western Blotting [2] 将HUVECs (5 × 104细胞/孔)接种于6孔板中,在5% CO2培养箱中37℃保存48小时。然后将培养基替换为无血清培养基,使细胞饥饿2.5小时。分别用不同浓度Homoplantaginin(0.1、1、10 μM)或Sal (500 μM)预处理细胞0.5 h,再用PA (100 μM)处理细胞3 h。此外,如上所述,对HUVECs进行播种和饥饿。然后,用Homoplantaginin(10 μM)或水杨酸盐(500 μM)预处理细胞0.5 h,再加脂多糖(1 μg/mL)预处理1 h。根据制造商推荐的膜和细胞质蛋白提取试剂盒获得相关蛋白裂解物,并用BCA蛋白测定试剂盒检测蛋白浓度。等量的蛋白质(30 μg)通过10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离,随后电转移到聚偏二氟乙烯膜上。在含有5%脱脂牛奶和0.05% Tween 20的tris缓冲盐水中37℃阻断2小时后,将膜与指定的抗体孵育。使用ChemiDoc XRS系统显示蛋白条带,并使用Image J软件(National Institutes of Health)进行分析。 ELISA法检测炎症介质[2] 将HUVECs (5 × 104细胞/孔)接种于6孔板中,在5% CO2培养箱中37℃保存48小时。然后将培养基替换为无血清培养基,使细胞饥饿2.5小时。分别用不同浓度的Homoplantaginin(0.1、1、10 μM)或Sal (500 μM)预处理细胞0.5 h,再用PA (100 μM)预处理细胞3 h。使用相应的ELISA试剂盒,按照制造商的说明,定量细胞上清液中IL-1β、ICAM-1和MCP-1的浓度。 细胞内一氧化氮水平测定[2] 将HUVECs (2 × 104个细胞/孔)接种于48孔板中,在5% CO2培养箱中37°C保存48小时。然后将培养基替换为无血清培养基,使细胞饥饿2.5小时。分别用不同浓度的Homoplantaginin(0.1、1、10 μM)或Sal (500 μM)预处理细胞0.5 h,再用PA (100 μM)预处理细胞3 h。然后用PBS洗涤细胞2次,并用5 μM, DAF-FM DA在37℃下孵育25分钟。用PBS洗涤2次后,在激发波长为495nm、发射波长为515nm的Olympus荧光显微镜下分析细胞。用Image J软件分析荧光强度。 |
| 动物实验 |
Rats: Homoplantaginin is dissolved in a solution of DMSO, PEG 400, ethanol, and normal saline (2:2:3:3, v/v/v/v) in rats at a concentration of 10 mg/mL. To administer oral administration (150 mg/kg), tail vein injection (15 mg/kg), and peritoneal injection (15 mg/kg) to the rats, the groups are randomly divided into three. At 5, 10, 20, 30, 45, 60, 90, 120, and 180 minutes after administration, blood samples (roughly 0.5 mL) are drawn from the retro-orbital plexus into heparinized microfugetubes. The plasma samples were extracted from the blood samples by centrifuging them at 10,000 rpm[4].
Mice: Homoplantaginin is dissolved in 5% amylum. Homoplantaginin is administered orally by gastric intubation during the experimental period at doses of 25, 50, 100 mg/kg/d, respectively. The mice are put to sleep with ether and blood samples are drawn by exsanguination from the inferior vein eight hours after LPS injection. To facilitate histological examination, the liver is taken out and fixed in formalin[1]. Acute liver injury model and Homoplantaginin treatment [1] The hepatoprotective effects of homoplantaginin were evaluated in Bacille-Calmette–Guérin and lipopolysaccharide (BCG/LPS)-induced immunological liver injury (ILI) in mice. Male Balb/c mice (20 ± 2 g) were housed in plastic cages with free access to food and water. BCG, 2.5 mg suspended in 0.2 ml saline, was injected through the tail vein in mice, and 10 d later they were injected with 7.5 μg lipopolysaccharide dissolved in 0.2 ml saline (LPS). Homoplantaginin was administered orally by gastric intubation during the experimental period at doses of 25, 50, 100 mg/kg/d, respectively. Bifendate, which is now clinically used for the treatment of hepatitis, was administered orally as a positive control (Pan et al., 2006). Eight hours after injection of LPS, the mice were anesthetized with ether and blood samples were collected by exsanguination from the inferior vein. The liver was removed and fixed in formalin for histological analysis (Lu et al., 2008, Wang et al., 2005b). Determination of apparent partition coefficient [4] The apparent partition coefficient of Homoplantaginin and hispidulin was measured by shake-flask method. Homoplantaginin and hispidulin was dissolved in different pH solutions. The resulted homoplantaginin and hispidulin solutions was mixed with n-octanol (presaturated with water) in equal volume, shaken, and incubated in a water bath at 37 °C for 24 h. After centrifugation, the concentrations of homoplantaginin and hispidulin in the aqueous phase and the octanol phase were determined by HPLC. Then the apparent partition coefficient was obtained by the ratio of the homoplantaginin and hispidulin concentrations in octanol phase to that in aqueous phase respectively. In situ single-pass intestinal perfusion(SPIP) studies in rats [4] The perfusion buffer consists of 1.4 g/L glucose, 20 mg/L phenol red, 7.8 g/L NaCl, 0.35 g/L KCl, 1.37 g/L NaHCO3, 0.32 g/L NaH2PO4, 0.02 g/L MgCl, 0.37 g/L CaCl2. Primary standard stock solution of Homoplantaginin was prepared in dimethylsulfoxide at 10 g/L. Working standard solutions of homoplantaginin were prepared by subsequent diluting primary standard stock solution with intestinal blank perfusion buffer. Briefly, male SD rats were fasted overnight for 16–18 h with free access to water and anaesthetized using an intra-peritoneal injection of 10% chloral hydrate(3.4 mL/kg) and placed on a heated pad to keep normal body temperature. Upon verification of the loss of pain reflex, a midline longitudinal abdominal incision was made, a 10 cm section of rat duodenum, jejunum, ileum, and colon were respectively located gently rinsed with saline pre-warmed to 37 °C and then attached to the perfusion assemblye. The entire surgical area was then covered with Parafilm to reduce evaporation. Blank perfusion buffer was infused for 30 min by a syringe pump followed by working standard solutions of Homoplantaginin at a constant flow rate of 0.2 mL/min for 120 min. The outlet perfusate samples was collected every 15 min in microtubes. At the end, the length of segment was measured without stretching and finally the animal was euthanatized [12]. Study samples were stored at −20 °C until analysis. Samples generated from the permeability study were analysed using validated method. Samples were centrifuged at 12,000 rpm for 10 min and the supernatant was directly injected onto HPLC column and required no sample preparation prior to analysis. Along with the permeability samples, QC samples were distributed in the analytical run. The data was accepted based on performance of QCs prepared using rat intestinal perfusion blank. Pharmacokinetic study [4] Homoplantaginin was dissolved in a solution consisting of dimethyl sulfoxide, PEG 400, ethanol and normal saline (2:2:3:3, v/v/v/v) at a concentration of 10 mg/mL. The rats were randomly divided into three groups to receive oral administration(150 mg/kg), tail vein injection (15 mg/kg) and peritoneal injectionv (15 mg/kg). Blood samples (approximately 0.5 mL) were collected from the retro-orbital plexus into heparinized microfuge tubes at 5, 10, 20, 30, 45, 60, 90, 120, and 180 min after administration. The plasma samples, separated by centrifuging the blood samples at 10,000 rpm for 1 min at 4 °C, were stored at −20 °C until further analysis. The following pharmacokinetic parameters were calculated by Phoenix_1.1 pharmacokinetic software according to non-compartmental model: the half-life (T1/2), area under the concentration-time curve (AUC), mean residence time (MRT), clearance (Cl), and the apparent volume of distribution at steady state (Vss). The data are presented in mean ± S.D. The maximum concentration (Cmax) and time of reaching maximum concentration (Tmax) were obtained from a concentration–time profile. The oral bioavailability (F) and intraperitoneal injection (Fi.p.) of bioavailability were calculated using the following equations: |
| 药代性质 (ADME/PK) |
Determination of apparent partition coefficient [4]
Hispidulin and Homoplantaginin were stable from pH 1.2 to pH 8.0 because the total concentration of the drug in both oil and water phase after 24 h incubation was not changed, suggesting that there are no degradation of hispidulin and homoplantaginin in gastrointestinal (GI) pH conditions. The results of apparent partition coefficient of hispidulin and homoplantaginin in different pH solutions are shown in Fig. 2. It can be observed that the apparent partition coefficients of hispidulin and homoplantaginin were ranged from 2.4 to 2.8 and 1.0–1.2. The results implied that hispidulin and homoplantaginin were not ionized in stomach or intestinal pH conditions and the absorption sites were stomach and intestine. It was also indicated by the results that the stomach and intestinal absorption of hispidulin and homoplantaginin independent of pH. In situ absorption of Homoplantaginin in rat intestinal segments [4] It has been reported that phenol red could interfere with the absorption and transport of poor water-soluble drugs in rat intestinal perfusion due to its partial absorption in the intestine [13]. Gravimetric method was used to calibrate the volume change of the perfusate. Effective permeabilities of homoplantaginin in four intestinal segments at three concentrations are presented in Table 1. The absorption of homoplantaginin depends on the intestinal site which could be found between duodenum, jejunum, ileum, and colon at each tested concentration. The effective permeabilities at three concentrations shared the same rank order of ileum > duodenum > jejunum > colon. The minimum and maximum Peff in rat intestinal segments were determined to be 0.259 × 10−4 (colon) and 0.687 × 10−4 cm/s (ileum). The permeability coefficients of homoplantaginin obtained from single-pass intestinal perfusion study were comparable to the reported values of propranolol (0.30–0.75 × 10−4 cm/s) [14], [15], [16], which was often used as a reference compound of passive absorption via transcellular pathway. Furthermore, there was no statistical difference of Peffs in four intestinal segments at each homoplantaginin concentration, suggesting the transport mechanism of homoplantaginin in four intestinal segments might be primarily passive transport. The absorption rate (Ka) of homoplantaginin showed the highest in jejunum, obviously decreased in duodenum and ileum, and then significantly reduced in icolon. Time course of the biotransformation of Homoplantaginin by rat intestinal bacteria in vitro [4] Fig. 3 shows the mean time-concentration curves of homoplantaginin in the biotransformation matrix of normal rat intestinal bacteria. As indicated in Fig. 3, homoplantaginin was metabolized fast, and almost completely biotransformation within 180 min, and 75% was transformed to hispidulin approximately at last. So, we know that in the case of the presence of intestinal bacteria, homoplantaginin can easily be converted into hispidulin, thus affecting its oral bioavailability. Pharmacokinetic results [4] The validated method was successfully applied to a pharmacokinetic study of Homoplantaginin. Fig. 4 shows the mean plasma concentration–time profiles of homoplantaginin after intravenous and peritoneal administration. Hispidulin and homoplantaginin were simultaneous quantified after oral administration in this study. Fig. 5 shows the mean plasma concentration–time profiles of homoplantaginin and hispidulin after oral administration. Based on the homoplantaginin and hispidulin plasma concentrations, the basic pharmacokinetic parameters were calculated and summarized in Table 2. As we can see after intravenous administration, the concentration of Homoplantaginin in plasma rapidly eliminated within 50 min. After peritoneal administration, it can be clearly seen that the drug concentration in plasma rapidly reached the peak value within 20 min with a mean Cmax between 15.45 and 21.99 nmol/mL and rapidly decreased during the next 100 min. The Fi.p.was calculated to be 102.85%. This indicated that homoplantaginin was absorbed completely in intestine as mentioned in determination of effective partition coefficient and in situ absorption in rat intestinal segments studies. Through the oral gavage, the Cmax of homoplantaginin was quickly reached among plasma after dosing due to fast absorption, with mean Tmax ranging from 7 to 25 min. The absolute oral bioavailability was calculated to be 0.75%. In comparison, hispidulin was rapidly absorbed (Tmax = 5–23 min), reaching a mean Cmax between 4.73 and 6.87 nmol/mL, the absolute bioavailability was 4.02%, which was about 5 times that of homoplantaginin. It was demonstrated that the majority of homoplantaginin were transformed to hispidulin by rat intestinal bacteria. As shown in Fig. 5, a double peak was observed in the concentration-time profile of oral administration. Several mechanisms might account for this phenomenon, such as enterohepatic circulation, fractionated gastric emptying, and separated “absorption windows”. Enterohepatic circulation may be an explanation for this because the double-peak was not observed in the IV concentration-time profile. Furthermore, the metabolites hispidulin presented the same phenomenon. [4] |
| 参考文献 |
|
| 其他信息 |
Homoplantaginin is a glycoside and a member of flavonoids.
Homoplantaginin has been reported in Salvia plebeia, Salvia officinalis, and other organisms with data available. Salvia plebeia R. Br is a traditional Chinese herb which has been considered as an inflammatory mediator used for treatment of many infectious diseases including hepatitis. Previously, the compound homoplantaginin was isolated in our group. Hence, we evaluated the protective effects of homoplantaginin on hepatocyte injury. Homoplantaginindisplayed an antioxidant property in a cell-free system and showed IC(50) of reduction level of DPPH radical at 0.35 microg/ml. In human hepatocyte HL-7702 cells exposed to H(2)O(2), the addition of 0.1-100 microg/ml of homoplantaginin, which did not have a toxic effect on cell viability, significantly reduced lactate dehydrogenase (LDH) leakage, and increased glutathione (GSH), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in supernatant. In vivo assay, we employed the model of Bacillus Calmette-Guérin (BCG)/lipopolysaccharide (LPS)-induced hepatic injury mice to evaluate efficacy of homoplantaginin. Homoplantaginin (25-100mg/kg) significantly reduced the increase in serum alanine aminotranseferase (ALT) and aspartate aminotransferase (AST), decreased the levels of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1 (IL-1). The same treatment also reduced the content of thiobarbituric acid-reactive substances (TBARS), elevated the levels of GSH, GSH-Px and SOD in hepatic homogenate. The histopathological analysis showed that the grade of liver injury was ameliorated with reduction of inflammatory cells and necrosis of liver cells in homoplantaginin treatment mice. These results suggest that homoplantaginin has a protective and therapeutic effect on hepatocyte injury, which might be associated with its antioxidant properties. [1] As a vasodilator and vascular homeostatic molecule, NO plays a vital role in regulating physiological endothelial function. However, bioactivity and generation of NO are usually impaired in inflamed endothelial cells.49 Our data indicated an impairment of NO production under PA challenge. As expected, we found that homoplantaginin restored the production of NO (Fig. 8). It indicated that Homoplantaginin could protect endothelial cells from PA insult by restoring impaired NO generation. CONCLUSION: In summary, our study elucidates that Homoplantaginin down-regulates TLR4 and NLRP3 inflammasome pathways, subsequently inhibits related inflammatory mediators, then restores impaired NO generation from PA-triggered endothelial cells. These findings suggest that homoplantaginin may potentially be a candidate agent for further development in the prevention and treatment of vascular diseases. [2] In summary, this study first report the pharmacokinetic of Homoplantaginin in rats after intravenous, peritoneal injection, and oral administration. Hispidulin and homoplantaginin were simultaneous quantified after oral administration for first time. The apparent partition coefficient of hispidulin and homoplantaginin were stable from pH 1.2 to pH 8.0 and homoplantaginin in intestinal segments might be primarily passive transport. The intestine is the best absorption site of homoplantaginin but it was almost completely biotransformation intermediate by rat intestinal bacteria. After oral administration, homoplantaginin was rapidly absorbed and the absolute oral bioavailability was 0.75%.[4] |
| 分子式 |
C22H22O11
|
|---|---|
| 分子量 |
462.4035
|
| 精确质量 |
462.116
|
| CAS号 |
17680-84-1
|
| 相关CAS号 |
17680-84-1
|
| PubChem CID |
5318083
|
| 外观&性状 |
Light yellow to yellow solid
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
798.1±60.0 °C at 760 mmHg
|
| 熔点 |
256-258℃
|
| 闪点 |
279.7±26.4 °C
|
| 蒸汽压 |
0.0±3.0 mmHg at 25°C
|
| 折射率 |
1.695
|
| LogP |
-0.97
|
| tPSA |
179.28
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
721
|
| 定义原子立体中心数目 |
5
|
| SMILES |
O1[C@]([H])([C@@]([H])([C@]([H])([C@@]([H])([C@@]1([H])C([H])([H])O[H])O[H])O[H])O[H])OC1C([H])=C2C(C(C([H])=C(C3C([H])=C([H])C(=C([H])C=3[H])O[H])O2)=O)=C(C=1OC([H])([H])[H])O[H]
|
| InChi Key |
GCLAFEGUXXHIFT-IWLDQSELSA-N
|
| InChi Code |
InChI=1S/C22H22O11/c1-30-21-14(32-22-20(29)19(28)17(26)15(8-23)33-22)7-13-16(18(21)27)11(25)6-12(31-13)9-2-4-10(24)5-3-9/h2-7,15,17,19-20,22-24,26-
|
| 化学名 |
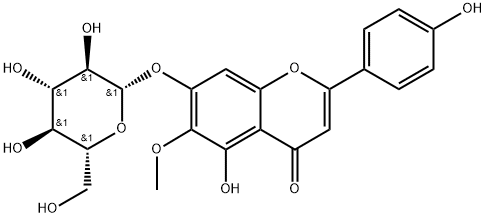
5-hydroxy-2-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one
|
| 别名 |
Homoplantaginin; 17680-84-1; HISPIDULOSIDE; Dinatin 7-glucoside; (-)-Homoplantaginin; hispidulin-7-glucoside; 4H-1-Benzopyran-4-one, 7-(beta-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxyphenyl)-6-methoxy-; 6-Methoxyapigenin 7-O-glucoside;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50~92 mg/mL (108.1~199.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (4.50 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1626 mL | 10.8131 mL | 21.6263 mL | |
| 5 mM | 0.4325 mL | 2.1626 mL | 4.3253 mL | |
| 10 mM | 0.2163 mL | 1.0813 mL | 2.1626 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|