| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| Other Sizes |
| 靶点 |
DNA Alkylator
DNA (alkylation and cross-linking; IC50 for human tumor cell lines: 20-100 μM, varies by cell type and exposure time) [1] - DNA replication and transcription (inhibition via DNA adduct formation) [2] |
|---|---|
| 体外研究 (In Vitro) |
Ifosfamide (50 mM) 增加肝细胞中 CYP3A4、CYP2C8 和 CYP2C9 蛋白水平,从而提高培养肝细胞中自身的 4-羟基化率。异环磷酰胺仅在一种人肝细胞培养物中诱导 CYP3A4,该培养物除了更广泛表达的 CYP3A4 之外还含有多态性表达的 CYP3A5。异环磷酰胺是一种前药,在肝脏中通过细胞色素 P450 混合功能氧化酶代谢为活性烷基化化合物异磷酰胺芥子。异环磷酰胺在小细胞肺癌、儿科实体瘤、非霍奇金和霍奇金淋巴瘤以及卵巢癌中产生了良好的缓解率。稳定转染 CYP2B1 后,异环磷酰胺对 MCF-7 细胞具有高度细胞毒性,但对亲代肿瘤细胞或表达 β-半乳糖苷酶的 MCF-7 转染子没有毒性,这种细胞毒性可以被 CYP2B1 抑制剂甲替拉酮明显阻断。小梁结构分析显示,异环磷酰胺与唑来膦酸联合使用比单独使用每种药物在预防肿瘤复发、改善组织修复和增加骨形成方面更有效。
72小时暴露后,抑制人非小细胞肺癌(NSCLC)细胞系(A549、H460)增殖,IC50分别为35 μM和42 μM;诱导G2/M期细胞周期阻滞和凋亡,表现为caspase-3活性升高和膜联蛋白V染色阳性[1] - 72小时处理对人卵巢癌细胞系SKOV3具有抗增殖活性,IC50为28 μM;50 μM浓度下克隆形成效率较未处理对照组降低65%[3] - 抑制人乳腺癌细胞系MCF-7的DNA合成;100 μM处理24小时后,因DNA交联导致[3H]-胸腺嘧啶掺入量减少80%[2] - 对顺铂耐药人膀胱癌细胞系T24具有细胞毒性,IC50为60 μM;与维生素E联合使用时活性增强,IC50降至32 μM[5] |
| 体内研究 (In Vivo) |
腹腔注射异环磷酰胺(100 mg/kg、200 mg/kg 和 400 mg/kg)可引起小鼠膀胱湿重和伊文思蓝外渗的剂量依赖性增加。异环磷酰胺显示小鼠广泛性膀胱炎,其特征是急性炎症,伴有血管充血、水肿、出血和纤维蛋白沉积、中性粒细胞浸润和上皮剥脱。异环磷酰胺对细胞质中的诱导型一氧化氮合酶表现出强烈的反应性,并且在苏木精和伊红染色中显示出强烈的弥漫性坏死。
抑制裸鼠A549 NSCLC异种移植瘤生长;每周静脉注射(i.v.)150 mg/kg,持续4周,肿瘤生长抑制率(TGI)达70%(相较于溶媒对照组)[1] - 抑制裸鼠人卵巢癌SKOV3异种移植瘤进展;每3天腹腔注射(i.p.)200 mg/kg,持续3个周期,肿瘤体积缩小65%,中位生存期延长12天[3] - 在大鼠原位膀胱癌模型中展现抗肿瘤活性;每周静脉注射100 mg/kg,持续3周,膀胱肿瘤重量降低58%,淋巴结转移灶减少[5] |
| 酶活实验 |
制备人肝微粒体以评估异环磷酰胺的代谢激活;将微粒体与10-100 μM 异环磷酰胺、NADPH再生系统和谷胱甘肽(GSH)在37°C下孵育60分钟;通过高效液相色谱(HPLC)定量活性代谢产物(异磷酰胺芥、丙烯醛);测定细胞色素P450(CYP)3A4和2B6依赖性代谢速率[2]
- 检测异环磷酰胺代谢产物的DNA交联活性;将小牛胸腺DNA与微粒体激活的异环磷酰胺(相当于50 μM母药)在37°C下孵育2小时;通过琼脂糖凝胶电泳分离交联DNA与单链DNA;采用光密度法量化交联效率[2] |
| 细胞实验 |
使用含有 2 毫升的培养基将 4 × 10 4 细胞接种到 3 cm 培养皿中。当达到终浓度时,第二天添加 0 至 5 mM 异环磷酰胺。除去培养基并用 PBS 清洁细胞并进行计数或染色后,还需要六天的时间[2]。
在96孔板中接种A549 NSCLC细胞,每孔2×103个;贴壁24小时后,用5-200 μM 异环磷酰胺处理72小时;采用MTT法测定细胞活力;计算IC50值,并通过碘化丙啶染色后流式细胞术分析细胞周期分布[1] - 在6孔板中培养SKOV3卵巢癌细胞,每孔5×103个;贴壁24小时后,用10-100 μM 异环磷酰胺处理48小时;洗涤细胞后在无药培养基中培养14天;甲醇固定并结晶紫染色;计数细胞数>50的克隆以确定克隆形成抑制率[3] - 在24孔板中接种T24膀胱癌细胞;用异环磷酰胺单独(20-100 μM)或与维生素E(10 μM)联合处理72小时;通过膜联蛋白V-FITC/PI双染色和流式细胞术检测凋亡细胞;使用发光试剂盒测定caspase-3/7活性[5] |
| 动物实验 |
Rats: Female rats are separated into four groups of eight before mating: group 1 is an untreated negative control group; group 2 is an injection of 1 mL of 0.9% NaCl; group 3 is an injection of 25 mg/kg Ifosfamide; and group 4 is an injection of 50 mg/kg Ifosfamide. Following five days of daily injections of Ifosfamide, three females are kept in a cage with one untreated male for a maximum of one week. Every day, vaginal smears are checked to see if someone is pregnant. In the event that sperm are found, the first 24 hours after mating are considered the first day of pregnancy. The expectant mothers are kept apart and regularly checked for symptoms of toxicity and miscarriage. On the eighteenth day of gestation, all pregnant animals are sacrificed by being beheaded. Serum is decanted and kept at -70°C until it is needed for the hormone assay. Cardiac blood (2.5–3 mL/rat) is collected in nonheparinized test tubes, centrifuged at 3,000× g for 30 min. The uterus and both ovaries are removed after blood collection, cleaned in saline solution, and the corpora lutea of pregnancy are counted visually. Each uterine horn is then examined to determine the number of viable fetuses, implantation sites, and resorption sites. Crown rump (CR) length is measured, weight is recorded, and each fetus is extracted from its umbilical cord. The placental weights are noted and the fetuses are inspected for external malformation. In order to facilitate histological and immunohistochemical analysis, fetuses and placentas from the control and treated groups are fixed in 10% neutral broth formalin.
Nude mice (6-7 weeks old) were implanted subcutaneously with 5×106 A549 NSCLC cells; when tumors reached 100 mm3, Ifosfamide was dissolved in normal saline and administered i.v. at 150 mg/kg once weekly for 4 weeks; control mice received normal saline; tumor volume was measured twice weekly with calipers, and tumor weight was recorded at sacrifice [1] - Female nude mice were implanted intraperitoneally with 1×107 SKOV3 ovarian cancer cells; 7 days post-implantation, Ifosfamide (dissolved in 5% dextrose solution) was given i.p. at 200 mg/kg every 3 days for 3 cycles; mice were monitored for survival, and peritoneal tumors were harvested to assess size and histopathology [3] - Rats with orthotopic bladder cancer (induced by N-butyl-N-(4-hydroxybutyl)nitrosamine) were randomized into treatment and control groups; Ifosfamide was dissolved in normal saline and administered i.v. at 100 mg/kg weekly for 3 weeks; control rats received normal saline; bladder tumors were excised and weighed, and lymph nodes were examined for metastases [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ifosfamide is extensively metabolized in humans and the metabolic pathways appear to be saturated at high doses. After administration of doses of 5 g/m2 of 14C-labeled ifosfamide, from 70% to 86% of the dosed radioactivity was recovered in the urine, with about 61% of the dose excreted as parent compound. At doses of 1.6–2.4 g/m2 only 12% to 18% of the dose was excreted in the urine as unchanged drug within 72 hours. Ifosfamide volume of distribution (Vd) approximates the total body water volume, suggesting that distribution takes place with minimal tissue binding. Following intravenous administration of 1.5 g/m2 over 0.5 hour once daily for 5 days to 15 patients with neoplastic disease, the median Vd of ifosfamide was 0.64 L/kg on Day 1 and 0.72 L/kg on Day 5. When given to pediatric patients, the volume of distribution was 21±1.6 L/m^2. 2.4±0.33 L/h/m^2 [pediatric patients] Renal excretion & t1/2 are dose & schedule dependent. 60-80% recovered as unchanged drug or metabolite in urine within 72 hr after admin. The distribution of ifosfamide (IF) and its metabolites 2-dechloroethylifosfamide (2DCE), 3-dechloroethylifosfamide (3DCE), 4-hydroxyifosfamide (4OHIF) and ifosforamide mustard (IFM) between plasma and erythrocytes was examined in vitro and in vivo. In vitro distribution was investigated by incubating blood with various concentrations of IF and its metabolites. In vivo distribution of IF, 2DCE, 3DCE and 4OHIF was determined in 7 patients receiving 9 g/m(2)/72 h intravenous continuous IF infusion. In vitro distribution equilibrium between erythrocytes and plasma was obtained quickly after drug addition. Mean (+/-sem) in vitro and in vivo erythrocyte (e)-plasma (p) partition coefficients (P(e/p)) were 0.75+/-0.01 and 0.81+/-0.03, 0.62+/-0.09 and 0.73+/-0.05, 0.76+/-0.10 and 0.93+/-0.05 and 1.38+/-0.04 and 0.98+/-0.09 for IF, 2DCE, 3DCE and 4OHIF, respectively. These ratios were independent of concentration and unaltered with time. The ratios of the area under the erythrocyte and plasma concentration--time curves (AUC(e/p)) were 0.96+/-0.03, 0.87+/-0.07, 0.98+/-0.06 and 1.34+/-0.39, respectively. A time- and concentration-dependent distribution--equilibrium phenomenon was observed with the relative hydrophilic IFM. It is concluded that IF and metabolites rapidly reach distribution equilibrium between erythrocytes and plasma; the process is slower for IFM. Drug distribution to the erythrocyte fraction ranged from about 38% for 2DCE to 58% for 4OHIF, and was stable over a wide range of clinically relevant concentrations. A strong parallelism in the erythrocyte and plasma concentration profiles was observed for all compounds. Thus, pharmacokinetic assessment using only plasma sampling yields direct and accurate insights into the whole blood kinetics of IF and metabolites and may be used for pharmacokinetic-pharmacodynamic studies. ... To assess the feasibility of a sparse sampling approach for the determination of the population pharmacokinetics of ifosfamide, 2- and 3-dechloroethyl-ifosfamide and 4-hydroxy-ifosfamide in children treated with single-agent ifosfamide against various malignant tumours. ... Pharmacokinetic assessment followed by model fitting. Patients: The analysis included 32 patients aged between 1 and 18 years receiving a total of 45 courses of ifosfamide 1.2, 2 or 3 g/m2 in 1 or 3 hours on 1, 2 or 3 days. ... A total of 133 blood samples (median of 3 per patient) were collected. Plasma concentrations of ifosfamide and its dechloroethylated metabolites were determined by gas chromatography. Plasma concentrations of 4-hydroxy-ifosfamide were measured by high-performance liquid chromatography. The models were fitted to the data using a nonlinear mixed effects model as implemented in the NONMEM program. A cross-validation was performed. ... Population values (mean +/- standard error) for the initial clearance and volume of distribution of ifosfamide were estimated at 2.36 +/- 0.33 L/h/m2 and 20.6 +/- 1.6 L/m2 with an interindividual variability of 43 and 32%, respectively. The enzyme induction constant was estimated at 0.0493 +/- 0.0104 L/h2/m2. The ratio of the fraction of ifosfamide metabolised to each metabolite to the volume of distribution of that metabolite, and the elimination rate constant, of 2- and 3-dechloroethyl-ifosfamide and 4-hydroxy-ifosfamide were 0.0976 +/- 0.0556, 0.0328 +/- 0.0102 and 0.0230 +/- 0.0083 m2/L and 3.64 +/- 2.04, 0.445 +/- 0.174 and 7.67 +/- 2.87 h(-1), respectively. Interindividual variability of the first parameter was 23, 34 and 53%, respectively. Cross-validation indicated no bias and minor imprecision (12.5 +/- 5.1%) for 4-hydroxy-ifosfamide only. ... We have developed and validated a model to estimate ifosfamide and metabolite concentrations in a paediatric population by using sparse sampling. ... The population pharmacokinetics and pharmacodynamics of the cytostatic agent ifosfamide and its main metabolites 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide were assessed in patients with soft tissue sarcoma. ... Twenty patients received 9 or 12 g/m2 ifosfamide administered as a 72-h continuous intravenous infusion. The population pharmacokinetic model was built in a sequential manner, starting with a covariate-free model and progressing to a covariate model with the aid of generalised additive modelling. ... The addition of the covariates weight, body surface area, albumin, serum creatinine, serum urea, alkaline phosphatase and lactate dehydrogenase improved the prediction errors of the model. Typical pretreatment (mean +/- SEM) initial clearance of ifosfamide was 3.03 +/- 0.18 l/h with a volume of distribution of 44.0 +/- 1.8 l. Autoinduction, dependent on ifosfamide levels, was characterised by an induction half-life of 11.5 +/- 1.0 h with 50% maximum induction at 33.0 +/- 3.6 microM ifosfamide. Significant pharmacokinetic-pharmacodynamic relationships (P = 0.019) were observed between the exposure to 2- and 3-dechloroethylifosfamide and orientational disorder, a neurotoxic side-effect. No pharmacokinetic-pharmacodynamic relationships between exposure to 4-hydroxyifosfamide and haematological toxicities could be observed in this population. For more Absorption, Distribution and Excretion (Complete) data for IFOSFAMIDE (6 total), please visit the HSDB record page. Metabolism / Metabolites Primarily hepatic. Ifosfamide is metabolized through two metabolic pathways: ring oxidation (\"activation\") to form the active metabolite, 4-hydroxy-ifosfamide and side-chain oxidation to form the inactive metabolites, 3-dechloro-ethylifosfamide or 2-dechloroethylifosfamide with liberation of the toxic metabolite, chloroacetaldehyde. Small quantities (nmol/mL) of ifosfamide mustard and 4-hydroxyifosfamide are detectable in human plasma. Metabolism of ifosfamide is required for the generation of the biologically active species and while metabolism is extensive, it is also quite variable among patients. Like cyclophosphamide, ifosfamide is activated in the liver by hydroxylation. However, the activation of ifosfamide proceeds more slowly, with greater production of dechlorinated metabolites & chloroacetaldehyde. These differences in metabolism likely account for the higher doses of ifosfamide required for equitoxic effects & the possible difference in antitumor spectrum of the two agents. Like cyclophosphamide, isophosphamide requires metabolism by microsomal enzymes to act as a cytotoxic agent. It is rapidly metabolized in many species, including rodents and dogs; the urinary metabolites indicate that a series of reactions take place analogous to those in the metabolism of cyclophosphamide. Acrolein is produced during its oxidative degradation, and one product of the reaction is the ring-opened carboxy derivative. Dogs also rapidly metabolize isophosphamide, and the carboxy derivative and 4-keto isophosphamide have been identified in the urine. The aim of this study was to develop a population pharmacokinetic model that could describe the pharmacokinetics of ifosfamide. 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide, and calculate their plasma exposure and urinary excretion. A group of 14 patients with small-cell lung cancer received a 1-h intravenous infusion of 2.0 or 3.0 g/m2 ifosfamide over 1 or 2 days in combination with 175 mg/m2 paclitaxel and carboplatin at AUC 6. The concentration-time profiles of ifosfamide were described by an ifosfamide concentration-dependent development of autoinduction of ifosfamide clearance. Metabolite compartments were linked to the ifosfamide compartment enabling description of the concentration-time profiles of 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide. The Bayesian estimates of the pharmacokinetic parameters were used to calculate the systemic exposure to ifosfamide and its metabolites for the four ifosfamide schedules. Fractionation of the dose over 2 days resulted increased metabolite formation, especially of 2-dechloroethylifosfamide, probably due to increased autoinduction. Renal recovery was only minor with 6.6% of the administered dose excreted unchanged and 9.8% as dechloroethylated metabolites. In conclusion, ifosfamide pharmacokinetics were described with an ifosfamide concentration-dependent development of autoinduction and allowed estimation of the population pharmacokinetics of the metabolites of ifosfamide. Fractionation of the dose resulted in increased exposure to 2-dechloroethylifosfamide, probably due to increased autoinduction. The anticancer drug ifosfamide is a prodrug requiring activation through 4-hydroxyifosfamide to ifosforamide mustard, to exert cytotoxicity. Deactivation of ifosfamide leads to 2- and 3-dechloroethylifosfamide and the release of potentially neurotoxic chloracetaldehyde. The aim of this study was to quantify and to compare the pharmacokinetics of ifosfamide, 2- and 3-dechloroethylifosfamide, 4-hydroxyifosfamide, and ifosforamide mustard in short (1-4 h), medium (24-72 h), and long infusion durations (96-240 h) of ifosfamide. An integrated population pharmacokinetic model was used to describe the autoinducible pharmacokinetics of ifosfamide and its four metabolites in 56 patients. The rate by which autoinduction of the metabolism of ifosfamide developed was found to be significantly dependent on the infusion schedule. The rate was 52% lower with long infusion durations compared with short infusion durations. This difference was, however, comparable with its interindividual variability (22%) and was, therefore, considered to be of minor clinical importance. Autoinduction caused a less than proportional increase in the area under the ifosfamide plasma concentration-time curve (AUC) and more than proportional increase in metabolite exposure with increasing ifosfamide dose. During long infusion durations dose-corrected exposures (AUC/D) were significantly decreased for ifosfamide and increased for 3-dechloroethylifosfamide compared with short infusion durations. No differences in dose-normalized exposure to ifosfamide and metabolites were observed between short and medium infusion durations. This study demonstrates that the duration of ifosfamide infusion influences the exposure to the parent and its metabolite 3-dechloroethylifosfamide. The observed dose and infusion duration dependence should be taken into account when modeling ifosfamide metabolism. Ifosfamide is a known human metabolite of L-trofosfamide. Primarily hepatic. Ifosfamide is metabolized through two metabolic pathways: ring oxidation (\"activation\") to form the active metabolite, 4-hydroxy-ifosfamide and side-chain oxidation to form the inactive metabolites, 3-dechloro-ethylifosfamide or 2-dechloroethylifosfamide with liberation of the toxic metabolite, chloroacetaldehyde. Small quantities (nmol/mL) of ifosfamide mustard and 4-hydroxyifosfamide are detectable in human plasma. Metabolism of ifosfamide is required for the generation of the biologically active species and while metabolism is extensive, it is also quite variable among patients. Route of Elimination: Ifosfamide is extensively metabolized in humans and the metabolic pathways appear to be saturated at high doses. After administration of doses of 5 g/m2 of 14C-labeled ifosfamide, from 70% to 86% of the dosed radioactivity was recovered in the urine, with about 61% of the dose excreted as parent compound. At doses of 1.6 - 2.4 g/m2 only 12% to 18% of the dose was excreted in the urine as unchanged drug within 72 hours. Half Life: 7-15 hours. The elimination half-life increase appeared to be related to the increase in ifosfamide volume of distribution with age. Biological Half-Life 7-15 hours. The elimination half-life increase appeared to be related to the increase in ifosfamide volume of distribution with age. The elimination half-life associated with doses of 2.5 g/sq m is 6-8 hr, whereas the elimination half-life associated with doses of 3.5-5 g/sq m is 14-16 hr. Undergoes extensive metabolism in the liver via CYP3A4 and CYP2B6 enzymes to form active metabolites (isophosphoramide mustard) and inactive metabolites (carboxyifosfamide, dechloroethylifosfamide) [2] - After i.v. administration of 150 mg/kg to rats, Ifosfamide had a plasma half-life (t1/2) of 1.5-2.0 hours; volume of distribution (Vd) was 0.6-0.8 L/kg [2] - Plasma protein binding rate was 15-20% in humans and rats; approximately 70% of the dose was excreted in urine within 24 hours, with 10-15% as active metabolites [2] - Oral bioavailability was <20% in dogs due to first-pass metabolism in the liver [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
After metabolic activation, active metabolites of ifosfamide alkylate or bind with many intracellular molecular structures, including nucleic acids. The cytotoxic action is primarily due to cross-linking of strands of DNA and RNA, as well as inhibition of protein synthesis. Hepatotoxicity The toxicity of ifosfamide seems to be similar to that of cyclophosphamide. Mild and transient elevations in serum aminotransferase levels are found in a high proportion of patients receiving ifosfamide. Because ifosfamide is typically given in combination with other antineoplastic agents, its role in causing the serum enzyme elevations is often not clear. The abnormalities are generally transient, do not cause symptoms and do not require dose modification. Clinically apparent liver injury from ifosfamide has been limited to a small number of cases of cholestatic hepatitis arising within a few weeks of receiving ifosfamide (with other antineoplastic agents). In addition, sinusoidal obstruction syndrome has been reported after conditioning regimens that have included ifosfamide in preparation for hematopoietic cell transplantation. The onset of injury is usually within one to three weeks of the myeloablation and is characterized by a sudden onset of abdominal pain, weight gain, ascites, marked increase in serum aminotransferase levels (and lactic dehydrogenase), and subsequent jaundice and hepatic dysfunction. The severity of sinusoidal obstruction syndrome varies from a transient, self limited injury to acute liver failure. The diagnosis is usually based on clinical features of tenderness and enlargement of the liver, weight gain, ascites and jaundice. Liver biopsy is diagnostic but often contraindicated, because of severe thrombocytopenia after bone marrow transplantation. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, especially alkylating agents such as ifosfamide. Labeling suggests that mothers should not breastfeed during therapy and for 1 week after the last dose of ifosfamide or mesna. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ifosfamide shows little plasma protein binding. Toxicity Data LD50 (mouse) = 390-1005 mg/kg, LD50 (rat) = 150-190 mg/kg. Interactions The more urotoxic agent ifosfamide was introduced to the market with the uroprotective agent, Mesna. Mesna liberated free thiol groups in the bladder which then can react with & neutralize the oxazaphosphorine metabolite. When administered in an appropriate dosing schedule, Mesna can prevent the bladder toxicity completely. BACKGROUND: The autoinducible metabolic transformation of the anticancer agent ifosfamide involves activation through 4-hydroxyifosfamide to the ultimate cytotoxic ifosforamide mustard and deactivation to 2- and 3-dechloroethylifosfamide with concomitant release of the neurotoxic chloroacetaldehyde. Activation is mediated by cytochrome P450 (CYP) 3A4 and deactivation by CYP3A4 and CYP2B6. The aim of this study was to investigate modulation of the CYP-mediated metabolism of ifosfamide with ketoconazole, a potent inhibitor of CYP3A4, and rifampin (INN, rifampicin), an inducer of CYP3A4/CYP2B6. METHODS: In a double-randomized, 2-way crossover study a total of 16 patients received ifosfamide 3 g/m(2) per 24 hours intravenously, either alone or in combination with 200 mg ketoconazole twice daily (1 day before treatment and 3 days of concomitant administration) or 300 mg rifampin twice daily (3 days before treatment and 3 days of concomitant administration). Plasma pharmacokinetics and urinary excretion of ifosfamide, 2- and 3-dechloroethylifosfamide, and 4-hydroxyifosfamide were assessed in both courses. Data analysis was performed with a population pharmacokinetic model with a description of autoinduction of ifosfamide. RESULTS: Rifampin increased the clearance of ifosfamide at the start of therapy at 102%. The fraction of ifosfamide metabolized to the dechloroethylated metabolites was increased, whereas exposure to the metabolites was decreased as a result of increased elimination. The fraction metabolized and the exposure to 4-hydroxyifosfamide were not significantly influenced. Ketoconazole did not affect the fraction metabolized or the exposure to the dechloroethylated metabolites, whereas both parameters were reduced with 4-hydroxyifosfamide. CONCLUSIONS: Coadministration of ifosfamide with ketoconazole or rifampin did not produce changes in the pharmacokinetics of the parent or metabolites that may result in an increased benefit of ifosfamide therapy. Leukopenic and/or thrombocytopenic effects of ifosfamide may be increased with concurrent or recent therapy if these medications /blood dyscarasia-causing medications/ cause the same effects; dosage adjustments of ifosfamide, if necessary, should be based on blood counts. Additive bone marrow depression may occur; dosage reduction may be required when two or more bone marrow depressants, including radiation, are used concurrently or consecutively /with ifosfamide/. For more Interactions (Complete) data for IFOSFAMIDE (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 143 mg/kg LD50 Rat ip 140 mg/kg LD50 Rat sc 160 mg/kg LD50 Rat iv 190 mg/kg For more Non-Human Toxicity Values (Complete) data for IFOSFAMIDE (8 total), please visit the HSDB record page. Dose-dependent hemorrhagic cystitis was observed in rats receiving i.v. doses >200 mg/kg weekly; characterized by bladder mucosal inflammation and hemorrhage, prevented by co-administration of mesna [2] - Myelosuppression (leukopenia, thrombocytopenia) occurred in nude mice at doses ≥150 mg/kg i.v.; nadir of white blood cell count was observed 7-10 days post-administration [1] - Neurotoxicity (ataxia, tremors) was reported in dogs receiving i.v. doses >250 mg/kg; associated with accumulation of chloroacetaldehyde, a toxic metabolite [2] - Mild nephrotoxicity (increased serum creatinine) was noted in rats treated with 150 mg/kg i.v. for 4 weeks; no significant hepatotoxicity was detected [4] - In vitro cytotoxicity to normal human fibroblasts (MRC-5) was low with IC50 >200 μM, indicating a therapeutic window [3] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Ifosfamide currently is approved for use in combination with other drugs for germ cell testicular cancer & is widely used to treat pediatric & adult sarcomas. Clinical trials also have shown ifosfamide to be active against carcinomas of the cervix & lung & against lymphomas. It is a common component of high-dose chemotherapy regimens with bone marrow or stem cell rescue; in these regimens, in total doses of 12-14 g/sq m, it may cause severe neurological toxicity, including coma & death. This toxicity is thought to result form a metabolite, chloracetaldehyde. In addition to hemorrhagic cystitis, ifosfamide causes nausea, vomiting, anorexia, leukopenia, nephrotoxicity, & CNS disturbances (especially somnolence & confusion). Ifosfamide is indicated, in combination with other antineoplastic agents and a prophylactic agent against hemorrhagic cystitis (such as mesna), for treatment of germ cell testicular tumors. /Included in US product labeling/ Ifosfamide is indicated as reasonable medical therapy for treatment of head and neck carcinoma. (Evidence rating: IIID) /NOT included in US product labeling/ Ifosfamide is used for treatment of soft-tissue sarcomas, Ewing's sarcoma, and Hodgkin's and non-Hodgkin's lymphomas. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for IFOSFAMIDE (9 total), please visit the HSDB record page. Drug Warnings It is a common component of high-dose chemotherapy regimens with bone marrow or stem cell rescue; in these regimens, in total doses of 12-14 g/sq m, it may cause severe neurological toxicity, including coma & death. This toxicity is thought to result form a metabolite, chloracetaldehyde. In addition to hemorrhagic cystitis, ifosfamide causes nausea, vomiting, anorexia, leukopenia, nephrotoxicity, & CNS disturbances (especially somnolence & confusion). Ifosfamide is distributed into breast milk. Breast feeding is not recommended during chemotherapy because of the risks to the infant (adverse effects, mutagenicity, carcinogenicity). The bone marrow depressant effects of ifosfamide may result in an increased incidence of microbial infection, delayed healing, and gingival bleeding. Dental work, whenever possible, should be completed prior to initiation of therapy or deferred until blood counts have returned to normal. Patients should be instructed in proper oral hygiene during treatment, including caution in use of regular toothbrushes, dental floss, and toothpicks. Many side effects of antineoplastic therapy are unavoidable and represent the medication's pharmacologic action. Some of these (for example, leukopenia and thrombocytopenia) are actually used as parameters to aid in individual dosage titration. For more Drug Warnings (Complete) data for IFOSFAMIDE (20 total), please visit the HSDB record page. Pharmacodynamics Ifosfamide requires activation by microsomal liver enzymes to active metabolites in order to exert its cytotoxic effects. Activation occurs by hydroxylation at the ring carbon atom 4 to form the unstable intermediate 4-hydroxyifosfamide. This metabolite than rapidly degrades to the stable urinary metabolite 4-ketoifosfamide. The stable urinary metabolite, 4-carboxyifosfamide, is formed upon opening of the ring. These urinary metabolites have not been found to be cytotoxic. N, N-bis (2-chloroethyl)-phosphoric acid diamide (ifosphoramide) and acrolein are also found. The major urinary metabolites, dechloroethyl ifosfamide and dechloroethyl cyclophosphamide, are formed upon enzymatic oxidation of the chloroethyl side chains and subsequent dealkylation. It is the alkylated metabolites of ifosfamide that have been shown to interact with DNA. Ifosfamide is cycle-phase nonspecific. Ifosfamide is an alkylating agent belonging to the oxazaphosphorine class, structurally related to cyclophosphamide [2] - Its antitumor effect is mediated by active metabolites that form intrastrand and interstrand DNA cross-links, inhibiting DNA replication and leading to cell death [1] - Approved for the treatment of various solid tumors, including testicular cancer, ovarian cancer, lung cancer, and bladder cancer [2] - Mesna is routinely used as a protective agent to prevent hemorrhagic cystitis by binding to acrolein, a toxic metabolite [2] - Shows activity against cyclophosphamide-resistant tumor cells due to differences in metabolic activation and DNA repair pathways [3] |
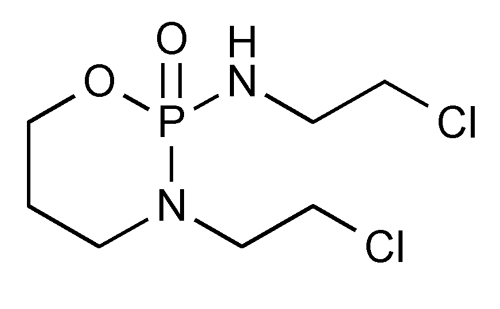
| 分子式 |
C7H15CL2N2O2P
|
|
|---|---|---|
| 分子量 |
261.09
|
|
| 精确质量 |
260.024
|
|
| 元素分析 |
C, 32.20; H, 5.79; Cl, 27.16; N, 10.73; O, 12.26; P, 11.86
|
|
| CAS号 |
3778-73-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
3690
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
336.1±52.0 °C at 760 mmHg
|
|
| 熔点 |
48ºC
|
|
| 闪点 |
157.1±30.7 °C
|
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
|
| 折射率 |
1.506
|
|
| LogP |
0.23
|
|
| tPSA |
51.38
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
218
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC([H])([H])C([H])([H])N1C([H])([H])C([H])([H])C([H])([H])OP1(N([H])C([H])([H])C([H])([H])Cl)=O
|
|
| InChi Key |
HOMGKSMUEGBAAB-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12)
|
|
| 化学名 |
N,3-bis(2-chloroethyl)-2-oxo-1,3,2lambda5-oxazaphosphinan-2-amine
|
|
| 别名 |
NSC-109724; Isophosphamide; Ifomide; NSC 109724; NSC109724; Iphosphamid; iphosphamide; Isoendoxan; IsoEndoxan; isophosphamide; Naxamide; Trade names: Cyfos; Ifex; Ifosfamidum
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 25 mg/mL (95.75 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8301 mL | 19.1505 mL | 38.3010 mL | |
| 5 mM | 0.7660 mL | 3.8301 mL | 7.6602 mL | |
| 10 mM | 0.3830 mL | 1.9150 mL | 3.8301 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Tafasitamab and Lenalidomide Followed by Tafasitamab and ICE As Salvage Therapy for Transplant Eligible Patients with Relapsed/ Refractory Large B-Cell Lymphoma
CTID: NCT05821088
Phase: Phase 2 Status: Recruiting
Date: 2024-11-15