| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
在脂肪细胞 (3T3-L1)、肝细胞 (HepG2) 和肌管 (C2C12) 的细胞模型中,吡虫啉可减少胰岛素刺激的过量用药。腺苷 B (AKT) 是胰岛素信号传导的主要控制者之一,在施用吡虫啉时磷酸化程度较低,但 AKT 的总体表达保持不变。核糖体 S6 (S6K) 是 AKT 的下游靶标和胰岛素信号传导的反馈放大器,在使用吡虫啉时磷酸化程度较低 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
增加吡虫啉剂量已被证明会降低认知功能,尤其是幼龄动物。这些影响可能归因于相关基因表达的改变。在2和8mg/kg剂量下,婴儿模型组的学习活动均显着降低;在 8 毫克/公斤水平下,学习活动进一步减少。此外,还发现 GRIN1、SYP 和 GAP-43 的表达水平没有明显变化 [2]。在斑马鱼中,吡虫啉的早期发育行为暴露会对大脑功能产生早期和长期的影响。整个生长阶段的吡虫啉处理显着减少了青春期幼虫对新池塘的探索,并增加了鱼类对惊吓刺激的运动感觉反应[3]。在20mg/kg/天时,体重增加量明显减少,并且在尸检、植入期间测量的相对体重在该水平上也显着增加。在最高剂量暴露下,自发运动活动以及血液学和体重指标均上升。高剂量的吡虫啉会引起肾脏、肝脏和大脑的退行性改变[4]。 20mg/kg剂量的吡虫啉显着改变心肌SOD、CAT、GPx、GSH和LPO;它还显着影响大脑 SOD、CAT 和 GPx 以及肾素 LPO [5]。高剂量时,吡虫啉可抑制细胞介导的免疫反应,如 DTH 反应降低和 PHA T 通路刺激指数降低所示。小鼠足垫切片的组织病理学研究表明,DTH 反应受到剂量相关的抑制;心脏和脾脏也出现了显着的组织病理学改变[6]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
/MILK/ [(14)C-methylene]Imidacloprid was administered to one 41 kg lactating goat by intubation in three consecutive daily doses of 10 mg/kg. The goat was sacrificed 2 hr after the last dose. The highest plasma concentration of 3.98 mg/mL was measured after 2 hr of last dosing. The highest radioactivity of 3.16-3.65 ug/g in the milk was determined 8 hr after the first dose and 2 hours after the third dose; the concentration in the milk prior to second dosing was 2.77 ug/g. Assuming a daily milk production of about 2 liters, the radioactivity in the milk was about 0.4% of the total administered radioactivity. The total residue in the edible tissues and organs measured two hours after the third dose was about 5% of the administered radioactivity. The respective residual radioactivity in the edible tissues was 1.3% (liver), (0.1%) kidney, (3%) muscles and 0.4% (fat). The main compounds in the milk and the edible tissues were imidacloprid, olefinic imidacloprid (NTN 35884) and 4- and 5-OH-imidacloprid. Five laying hens were intubated with 10 mg/kg methylene-labeled- (14)C-imidacloprid for 3 days. The highest radioactivity of 0.34 ug/mL in the plasma was measured at 0.5 hr after the third dosing. At that time, the total residue in the edible tissues and organs was about 3% of the total dose. The highest radioactivity of 1.347 ug/g in eggs was found 2 hr after the last dose. This level was less than 0.2% of the total administered radioactivity. The main metabolite in the eggs was the olefine-imidacloprid. Olefine- and desnitro-imidacloprid were detected in muscle and kidney tissues. A: NTN 33893, 99.9% purity), B: 1-[(6-chloro-3-pyridinyl) (14)C-methyl]-4,5dihydro- N-nitro-1H-imidazol-2-amine] (150.7 uCi/mg, >99% purity); oral: single (1 mg/kg B, 20 mg/kg B), multiple (1 mg/kg daily for 14 days followed by 24 hr after final dose by a single dose of B); IV: single (1 mg/kg B); 5 rats/sex/dose; following oral and intravenous administration 94 - 100% of the administered radioactivity is absorbed and readily distributed to the body from the central compartment as indicated by a short mean absorption half-life (35 minutes) and an apparent volume of distribution accounting for about 84% of the total body volume; the small mean residence time which vary between 9 and 17 hours suggests that the total radioactivity is rapidly eliminated from the body; after oral or intravenous administration, 91.4 to 96% of the given dose was excreted via urine and feces by 48 hours; no significant amount of radioactivity was found in expired air; high concentrations of total radioactivity were observed in the kidney, liver, lung and skin; no signs of bioaccumulation were evident. [Imidazolidine-4,5-(14)C] Imidacloprid (0.827 uCi/mg, 99.8% purity, ... and 124 uCi/mg, >99% purity ... ); oral; 1 mg/kg (10 male /rats/, 5 female /rats/) and 150 mg/kg (5 male /rats/); absorption after oral dosing is rapid and maximal plasma concentration is achieved between 1 and 1.5 hr at the low dose and 4 hr at the high dose; after oral administration of the imidazolidine labeled compound, the renal-excreted portion of the given dose is higher (91%) as compared to methylene labeled Imidacloprid (75%); fecal elimination plays a minor role and 1% of the administered radioactivity remains in the body at 48 hr; highest radioactivity concentrations were reported in liver irrespective of dose level; 5 metabolites were identified in urine which represent 77% of radioactivity recovered in urine. For more Absorption, Distribution and Excretion (Complete) data for IMIDACLOPRID (10 total), please visit the HSDB record page. Metabolism / Metabolites A: NTN 33893, 99.9% purity), B: 1-[(6-chloro-3pyridinyl)(14)C-methyl]-4,5-dihydro-N-nitro-(1)H-imidazol-2-amine] (150.7 uCi/mg, >99% purity); oral: single (1 mg/kg B, 20 mg/kg B), multiple (1 mg/kg A qd @ 14 days followed by 24 hrs after final dose by a single dose of B); IV: single (1 mg/kg B); 5 rats/sex/dose; >90% of administered radioactivity eliminated 48 hours after dosing with less than 1% remaining in the carcass in all dose groups; no sex differences in excretion pattern and metabolic profiles of the excreta were evident following low dose administration; however, at the high dose, females showed a slightly higher renal elimination rate than males; males showed a higher capacity to metabolize the test compound and the amount of parent compound was lower as compared to females; oxidation cleavage of the parent compound yields 6-chloronicotinic acid which then reacts with glycine to form a conjugate (WAK 3583); second major route of metabolism involves hydroxylation and elimination of water from the imidazolidine ring in the 4- or 5-position to produce the metabolite NTN 35884; these metabolites are excreted in urine and feces; no evidence of bioaccumulation following multiple dosing was reported. methylene-[(14)C] imidacloprid (86.4 - 123 uCi/mg, 98.4 - 99% purity): single [1 mg/kg (5 males), 150 mg/kg (7 males)] and chronic treatment with the unlabeled imidacloprid for 1 year in diet prior to receiving radiolabeled imidacloprid (80 mg/kg, 10 males); methylene-[(14)C] WAK 3839 (40 uCi/mg, 99% purity): 1 mg/kg 5 males); both compound absorbed rapidly after single oral dosing; terminal half-lives for imidacloprid and WAK 3839 are 35.7 and 46.9 hrs, respectively; 75% of the given dose of both compounds are eliminated primarily via urine within 48 hrs; fecal elimination plays a minor role, since 21% and 16% of the recovered radioactivity are excreted by this route, respectively; glycine conjugate of 6-Cl-nicotinic acid (WAK 3583), two monohydroxylated metabolites (WAK 4103) and the unsaturated metabolite (NTN 35884) were identified in the urine and accounted for 82% of the total radioactivity; same metabolites were also identified in feces; besides unchanged WAK 3839, one other metabolite NTN 33823 was identified in urine and feces obtained from rats treated with WAK 3839; WAK 3839 and other metabolites identified after a single low dose were detected in urine from rats and mice treated chronically with imidacloprid in their diet; this finding suggests that WAK 3839 is formed during chronic exposure to imidacloprid. WAK 3839, a metabolite of NTN 33893; 98.9% purity; /V79-HGPRT assay with/ doses (based on solubility limit and cytotoxicity test): 500, 1000, 1500, 1750, & 2000 ug/mL for both -S9 trials and 1 of 2 +S9 trials; for the other +S9 trial the doses were 500, 750, 1000, 1250, 1500, & 1750 ug/mL; after plating 4 x 106 cells/250 mL flask, the cells were exposed to test article (-/+ S9 microsomes) for 5 hr followed by an "expression period" of exponential growth and subsequent replating under selective conditions (10 ug/mL 6thioguanine) at 3 x 105 cells/100 mm dish; after 7 days the colonies were fixed and counted; duplicate exposure dishes were run, each dish generating 8 replicate dishes in the selection condition; test article did not induce 6-thioguanine resistance at any dose despite success of positive controls (-S9, ethyl methane sulfonate; +S9, DMBA); it is not mutagenic in this system under these conditions. WAK 3839, a metabolite of NTN 33893; 94.3% purity; /CHO-HGPRT assay with/ doses (based on solubility limit and cytotoxicity test), -S9: 62.5, 125, 250, 500, 1000, & 2000 ug/mL; +S9: 500, 750, 1000, 1250, 1500, & 2000; after plating 4 x 106 cells/250 mL flask, the cells were exposed to test article (-/+ S9 microsomes) for 5 hr followed by an "expression period" of exponential growth and subsequent replating under selective conditions (10 ug/mL 6-thioguanine) at 3 x 105 cells/100 mm dish; after 7 days the colonies were fixed and counted; duplicate exposure dishes were run, each dish generating 8 replicate dishes in the selection condition; test article did not consistently induce 6-thioguanine resistance at any dose despite success of positive controls (-S9, ethyl methane sulfonate; +S9, DMBA); it is not mutagenic in this system under these conditions. For more Metabolism/Metabolites (Complete) data for IMIDACLOPRID (14 total), please visit the HSDB record page. Imidacloprid has known human metabolites that include olefin, 5-hydroxy-imidacloprid, and 1H-Imidazol-2-amine, 1-[(6-chloro-3-pyridinyl)methyl]-4,5-dihydro-N-nitroso-. Biological Half-Life The half-lives for excretion of the radiolabeled imidacloprid were calculated in rats after a single i.v. dose of 1 mg/kg, after single oral doses of 1 and 20 mg/kg or after multiple doses of 1 mg/kg. The excretion half-life values varied greatly (from 26 hr to 118 hr), but the variation was not dose-, sex-, or route-dependent. ... methylene-[(14)C] imidacloprid (86.4 - 123 uCi/mg, 98.4 - 99% purity): single [1 mg/kg (5 males), 150 mg/kg (7 males)] and chronic treatment with the unlabeled imidacloprid for 1 year in diet prior to receiving radiolabeled imidacloprid (80 mg/kg, 10 males); methylene-[(14)C] WAK 3839 (40 uCi/mg, 99% purity): 1 mg/kg 5 males); both compound absorbed rapidly after single oral dosing; terminal half-lives for imidacloprid and WAK 3839 are 35.7 and 46.9 hrs, respectively ... . ... Honeybees were treated orally with imidacloprid at 20 and 50 ug/kg per bee. ... Imidacloprid had a half-life ranging between 4.5 and 5 hr and was rapidly metabolized into 5-hydroxyimidacloprid and olefin. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Imidacloprid (IM) forms colorless crystals. It is registered for pesticide use in the USA but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. IM is used to control insect pests on agricultural and nursery crops, structural pests and parasites on companion animals. HUMAN EXPOSURE AND TOXICITY: The most common clinical signs included: rash, breathing difficulty, headache, tearing eyes, nausea, itching, dizziness, increased salivation, vomiting, numbness and dry mouth. One case was reported for a worker who had imidacloprid splashed into the eyes. The clinical signs were burning and corneal abrasion in the eye. IM blood concentrations found in two fatal cases were 12.5 and 2.05 ug/mL. The damage induced by imidacloprid in the HepG2 cells resulted from a clastogenic action of this insecticide (76.6% of the MN /micronucleus test/ did not present a centromeric signal). ANIMAL STUDIES: IM (purity, 94.2%) did not irritate the eyes or skin of rabbits and did not sensitize the skin of guinea-pigs. IM given orally as a single dose was moderately toxic to rats and mice. Behavioral and respiratory signs, disturbances of motility, narrowed palpebral fissures, transient trembling and spasms were seen in rats and mice treated orally at doses /greater than or equal/ to 200 mg/kg bw and /greater than or equal to/ 71 mg/kg bw, respectively. The clinical signs were reversed within 6 days. In chronic experiments conducted in rats, the liver was the principal target organ, with hypertrophy of hepatocytes and sporadic cell necrosis in high-dose males only. Liver pathology was mild at termination of the study and was fully reversible within the recovery period. IM-treated male rats showed histopathological alterations in testis and epididymis. In developmental studies in rats, there was a high percentage of male fetuses and an increased incidence of wavy ribs was observed. In a developmental rabbit study, fecundity was decreased at the high dose based on observed abortions, total litter resorptions, and increased post-implantation loss due to increased late resorptions. However, this dose level also resulted in decreases in body weight and body weight gain and produced an increase in mortality. Early developmental exposure to IM has both early-life and persisting effects on neurobehavioral function in zebrafish. Rats teated in vivo with 170 mg/kg IM, structural chromosome aberrations, abnormal cells and mitotic index were determined microscopically in bone marrow cells. Male rats in particular showed susceptibility to the genotoxic effects of imidacloprid. ECOTOXICITY STUDIES: IM effect on beneficial insects such as the honeybee Apis mellifera L is still controversial. IM administered at levels found in agroecosystems can reduce sensitivity to reward and impair associative learning in young honeybees. Therefore, once a nectar inflow with IM traces is distributed within the hive, it could impair in-door duties with negative consequences on colony performance. When laboratory-reared adult worker honey bees were treated with sublethal doses of IM, neuronal apoptosis was detected using the TUNEL technique for DNA labeling. Behavioral effects of IM and 5-OH-IM were studied using the olfactory conditioning of proboscis extension response at two periods of the year. Winter bees surviving chronic treatment with IM and 5-OH-IM had reduced learning performances. The lowest observed effect concentrations of IM was lower in summer bees (12 ug/kg) than in winter bees (48 ug/kg), which points to a greater sensitivity of honeybees behavior in summer bees, compared to winter bees. Oral acute and chronic toxicity of IM and its main metabolites (5-hydroxyimidacloprid, 4,5-dihydroxyimidacloprid, desnitroimidacloprid, 6-chloronicotinic acid, olefin, and urea derivative) were investigated in Apis mellifera. Acute intoxication by IM or its metabolites resulted in the rapid appearance of neurotoxicity symptoms, such as hyperresponsiveness, hyperactivity, and trembling and led to hyporesponsiveness and hypoactivity. Bumblebees (Bombus terrestris audax) colonies exposed to IM show deficits in colony growth and nest condition compared with untreated colonies. In mallard duck reproductive studies, effects on eggshell thickness were observed at concentrations of greater than or equal to 61 mg/kg-diet; a 52% decrease in female body weight gain was reported at 241 ppm. In the fish early-life cycle study with rainbow trout, treatment-related decreases in growth and survival were noted at concentrations of greater than or equal to 1.2 mg a.i./L. Toxicity Data LC50 (rat) > 5,323 mg/m3/4h Interactions Standard ecotoxicological risk assessments are conducted on individual substances, however monitoring of streams in agricultural areas has shown that pesticides are rarely present alone. In fact, brief but intense pulse events such as storm water runoff and spray drift during application subject freshwater environments to complex mixtures of pesticides at high concentrations. This study investigates the potential risks to non-target aquatic organisms exposed to a brief but intense mixture of the neonicotinoid pesticides imidacloprid and thiacloprid and the pyrethroid pesticides deltamethrin and esfenvalerate, compared to single substance exposure. All four of these pesticides have been detected in surface waters at concentrations higher than benchmark values and both classes of pesticides are known to exert adverse effects on non-target aquatic organisms under single substance exposure scenarios. First instar midge larvae of the non-target aquatic organism, Chironomus riparius, were exposed to combinations of these four pesticides at 50% of their LC50 (96 hr) values in a 1 hr pulse. They were then reared to adulthood in uncontaminated conditions and assessed for survival, development time and fecundity. Our results show that the risk of disruption to survival and development of non-target aquatic organisms under this scenario is not negligible on account of the significant increases in mortality of C. riparius found in the majority of the pesticide exposures and the delays in development after pyrethroid exposure. While none of the deleterious effects appear to be amplified by combination of the pesticides, there is some evidence for antagonism. No effects on fecundity by any of the pesticide treatments were observed. Earlier research has evidenced the oxidative and neurotoxic potential of imidacloprid, a neonicotinoid insecticide, in different animal species. The primary aim of this study was to determine how metabolic modulators piperonyl butoxide and menadione affect imidacloprid's adverse action in the liver and kidney of Sprague-Dawley rats of both sexes. The animals were exposed to imidacloprid alone (170 mg/kg) or in combination with piperonyl butoxide (100 mg/kg) or menadione (25 mg/kg) for 12 and 24 hr. Their liver and kidney homogenates were analyzed spectrophotometrically for glutathione peroxidase, glutathione S-transferase, catalase, total cholinesterase specific activities, total glutathione, total protein content, and lipid peroxidation levels. Imidacloprid displayed its prooxidative and neurotoxic effects predominantly in the kidney of male rats after 24 hr of exposure. Our findings suggest that the observed differences in prooxidative and neurotoxic potential of imidacloprid could be related to differences in its metabolism between the sexes. Co-exposure (90-min pre-treatment) with piperonyl butoxide or menadione revealed tissue-specific effect of imidacloprid on total cholinesterase activity. Increased cholinesterase activity in the kidney could be an adaptive response to imidacloprid-induced oxidative stress. In the male rat liver, co-exposure with piperonyl butoxide or menadione exacerbated imidacloprid toxicity. In female rats, imidacloprid+menadione co-exposure caused prooxidative effects, while no such effects were observed with imidacloprid alone or menadione alone. In conclusion, sex-, tissue-, and duration-specific effects of imidacloprid are remarkable points in its toxicity. The combined toxicity of five insecticides (chlorpyrifos, avermectin, imidacloprid, lambda-cyhalothrin, and phoxim), two herbicides (atrazine and butachlor) and a heavy metal (cadmium) has been examined with the earthworm acute toxicity test. Toxicological interactions of these chemicals in four, five, six, seven, and eight-component mixtures were studied using the combination-index (CI) equation method. In four-component and five-component mixtures, the synergistic effects predominated at lower effect levels, while the patterns of interactions found in six, seven, and eight-component mixtures displayed synergism. The lambda-CY+IMI+BUT+ATR+CPF+PHO combination displayed the most strongly synergistic interaction, with CI values ranging from 0.09 to 0.15. The nature of the interaction changes with the effect level and the relevance of synergistic effects increase with the complexity of the mixture. The CI method was compared with the classical models of concentration addition (CA) and independent action (IA) and we found that the CI method could accurately predict the combined toxicity. The predicted synergism resulted from co-existence of the pesticides and the heavy metal especially at low effect levels may have important implications in risk assessment for the real terrestrial environment. Metabolic modifiers and other pharmaceutical drugs have been shown to modify the toxicity of imidacloprid. The CYP450-inhibiting piperonyl butoxide synergized the toxicity of imidacloprid. In subchronic and chronic feeding studies, mice developed hypersensitivity to ether, which was used as anesthesia during procedures such as blood withdrawal and tattooing. These animals exhibited dyspnea, respiratory failure and spasms and died shortly after administration of ether. The specific mechanism of the imidacloprid-induced hypersensitivity to ether is presently unknown. For more Interactions (Complete) data for IMIDACLOPRID (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat (male) oral 424 mg/kg LD50 Rat (female) oral 450-475 mg/kg LD50 Mouse (male) oral 131 mg/kg LD50 Mouse (female) oral 168 mg/kg For more Non-Human Toxicity Values (Complete) data for IMIDACLOPRID (18 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
(E)-imidacloprid is the E-isomer of imidacloprid.
Imidacloprid is a neonicotinoid, which is a class of neuro-active insecticides modeled after nicotine. Imidacloprid is a patented chemical, Imidacloprid is manufactured by Bayer Cropscience (part of Bayer AG) and sold under trade names Kohinor, Admire, Advantage, Gaucho, Merit, Confidor, Hachikusan, Premise, Prothor, and Winner. It is marketed as pest control, seed treatment, an insecticide spray, termite control, flea control, and a systemic insecticide. See also: Imidacloprid; Moxidectin (component of); Imidacloprid; Ivermectin (component of). Therapeutic Uses Cholinergic Agents; Insecticides /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Imidacloprid is included in the database. (VET): Ectoparasiticide. |
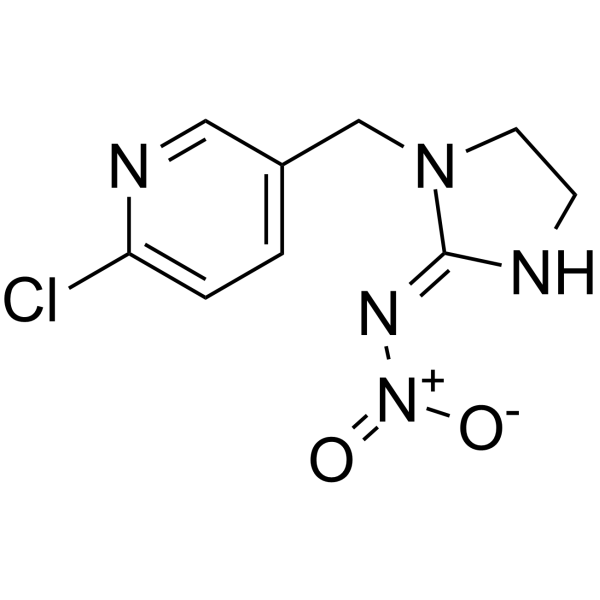
| 分子式 |
C9H10CLN5O2
|
|---|---|
| 分子量 |
255.66
|
| 精确质量 |
255.052
|
| CAS号 |
138261-41-3
|
| 相关CAS号 |
Imidacloprid-d4;1015855-75-0
|
| PubChem CID |
86287518
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
442.3±55.0 °C at 760 mmHg
|
| 熔点 |
144ºC
|
| 闪点 |
221.3±31.5 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.706
|
| LogP |
-0.43
|
| tPSA |
89.04
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
319
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1CN(/C(=N/[N+](=O)[O-])/N1)CC2=CN=C(C=C2)Cl
|
| InChi Key |
YWTYJOPNNQFBPC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H10ClN5O2/c10-8-2-1-7(5-12-8)6-14-4-3-11-9(14)13-15(16)17/h1-2,5H,3-4,6H2,(H,11,13)
|
| 化学名 |
(NE)-N-[1-[(6-chloropyridin-3-yl)methyl]imidazolidin-2-ylidene]nitramide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~391.14 mM)
H2O : ~1 mg/mL (~3.91 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.78 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.78 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.78 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9114 mL | 19.5572 mL | 39.1144 mL | |
| 5 mM | 0.7823 mL | 3.9114 mL | 7.8229 mL | |
| 10 mM | 0.3911 mL | 1.9557 mL | 3.9114 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05179005 | Terminated | Device: RibFix Advantage | Rib Fractures | Zimmer Biomet | 2023-04-20 | |
| NCT04163224 | Withdrawn | Device: RibFix Advantage | Rib Fracture Multiple | Zimmer Biomet | 2020-04-01 | |
| NCT04184271 | Completed | Drug: 38% silver diamine fluoride | Dental Caries | Advantage Silver Dental Arrest, LLC | 2017-08-01 | Phase 2 |
| NCT04186663 | Completed | Drug: Silver Diamine Fluoride | Dental Caries | Advantage Silver Dental Arrest, LLC | 2019-08-01 | Phase 2 |
| NCT02645617 | Completed | Drug: Varnish | Dental Caries | Advantage Dental Services, LLC | 2016-03-30 | Phase 1 |
|
|