| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT6 Receptor ( pKi = 9.63 )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:SB-742457 是一种选择性 5-HT6 受体拮抗剂,具有认知、记忆和学习增强作用。 SB-742457 是一种治疗阿尔茨海默病的新型认知增强剂。 SB-742457 是一种潜在药物,可稳定轻度至中度阿尔茨海默病受试者的多奈哌齐治疗。
|
| 体内研究 (In Vivo) |
SB-742457 是一种有效的选择性 5-HT6 受体拮抗剂,可逆转新物体识别测试中东莨菪碱诱导的学习缺陷,并提高老年大鼠在水迷宫任务中的表现。
本研究旨在研究所选APD(氟哌啶醇、利培酮、奥氮平)单独或与选择性5-HT6激动剂(WAY-181187)或拮抗剂(Intepirdine (SB742457))联合给药对大鼠体重增加、食物摄入、血脂谱、葡萄糖水平以及脂肪(瘦素、脂联素)和胃肠道(胰岛素、胃饥饿素)组织衍生激素谱的慢性影响。SB-742457比WAY-181187更能抑制APD引起的体重增加和缓解高血糖,但也会加剧血脂异常。WAY-181187倾向于改善脂质状况,但增加了葡萄糖水平。当WAY-181187或英特哌定(SB742457)与氟哌啶醇联合给药时,获得了最大的益处。很难评估血清胰岛素、瘦素、胃饥饿素和脂联素水平的改变是否取决于所应用的治疗或其他药物非依赖性因素;因此,需要进一步的研究。[2] 本研究旨在调查和比较急性和慢性(21天)给予5-羟色胺6受体(5-HT6R)激动剂(WAY-181187)和拮抗剂(Intepirdine(SB742457))对MK-801诱导的新物体识别(NORT)和Y迷宫连续自发交替试验(Y-CAT)记忆障碍的影响。此外,在给药21天后测量大鼠海马中脑源性神经营养因子(BDNF)的表达,以研究BDNF参与5-HT6R配体的促认知作用。我们发现,WAY-181187和SB-742457的急性给药逆转了MK-801在NORT和Y-CAT中的作用,并且这种影响在NORT中长期应用后仍然存在,但在Y-CAT中没有。两种5-HT6R配体都增加了海马BDNF蛋白的表达,但WAY-181187比SB-742457更有效,更好地缓解了MK-801诱导的BDNF信号通路的抑制,这似乎在行为测试中转化为更强的WAY-181187-效应。总的来说,急性和慢性给药的5-HT6R激动剂和拮抗剂都可以预防MK-801诱导的大鼠记忆障碍和BDNF信号的改变。目前的结果证实了这两种5-HT6R配体的促认知特性,并表明BDNF通路可能参与了它们的作用机制[3]。 |
| 酶活实验 |
第2组(n=6)用于11C-GSK215083区域结合的药理学表征。除了对5HT6受体的亲和力外,PET配体11C-GSK215083对5HT2A受体的亲和力也低约5倍(3)。因此,我们使用选择性5HT2A拮抗剂酮色林来检测是否存在与5HT2A受体的可检测结合,并使用SIntepirdine(SB742457)来检测与5HT6受体的结合。四名受试者分别在SB742457(口服175 mg,11C-GSK215083给药前5小时)和酮色林(缓慢静脉注射0.1 mg/kg,11C-GSK215083给药前2小时)后,以随机方式进行了基线11C-GSK251083扫描,然后进行了2次PET扫描,间隔7天。其余2名受试者接受了基线扫描、SB742457(175 mg)后的第二次扫描和7天后的第三次扫描(图1B)。
第3组(n=8)描述了英特哌定(SB742457)在大脑中的时间和剂量占用关系。每位受试者接受了3次PET扫描。扫描1是基线扫描,扫描2和3是在给药7至28天期间,每天重复一次SB742457给药2次后进行的。4名受试者在第1天接受了SB742457(175 mg)的负荷剂量,随后每天服用35 mg,持续21或28天(每组2名受试人)。受试者被随机分配在第7、14、21和28天接受2次扫描,每个时间点收集2个独立的数据点(图1C)。同样,另外2名受试者接受了SB742457(70 mg)治疗,随后每天服用15 mg,持续14天,最后2名受试验者接受了15 mg治疗,随后每日服用3 mg,连续14天。所有4名受试人都在第7天和第14天接受了扫描(图1D)。SB742457给药后约5小时进行扫描。 安全性评估包括病史、体检、不良事件报告、临床实验室评估和心电图、血压和脉搏率测量(补充信息)。在每次扫描之前和完成时,从第2组和第3组的受试者身上采集血液样本,用于血浆中SB742457的药代动力学分析,并给予英特哌定(SB742457)(补充信息)。https://pubmed.ncbi.nlm.nih.gov/26383152/ |
| 细胞实验 |
蛋白质印迹分析[3]
使用含有蛋白酶和磷酸酶抑制剂的T-PER哺乳动物蛋白提取试剂对海马样本进行均质化。使用Bradford反应测定蛋白质浓度。将等分试样(40μg)溶解在含有2%2-巯基乙醇的Laemmli缓冲液中,并进行10%SDS-聚丙烯酰胺凝胶电泳。使用1:500稀释的抗脑源性神经营养因子(BDNF,15-kDa)和1:1000稀释的抗β-肌动蛋白。第二抗体为1:2000稀释的抗兔IgG(HRP)。 定量实时PCR[3] 使用RNA分离试剂盒从组织中提取RNA。在数量和质量评估后,RNA浓度标准化为15 ng/µL。使用高容量逆转录试剂盒进行逆转录。根据制造商在Applied Biosystems®7500快速实时PCR仪上的方案,使用TaqMan BDNF引物和探针(Rn02531967_s1)进行qPCR 96孔反应板。在初步实验的基础上,选择了内源性控制基因Gapdh(Rn01775763_g1)和Tbp(Rn01455646_m1)。使用ΔΔCq方法计算相对表达式。 |
| 动物实验 |
A total of 140 rats were used in the study, and each treatment group consisted of 10 randomly selected animals. Due to the large number of animals and limited laboratory space, the experiment was carried out in three turns: the first included 4 treatment groups: vehicle (1% Tween 80), haloperidol, risperidone, and olanzapine; the second consisted of 5 treatment groups: vehicle (1% Tween 80), WAY-181187, haloperidol + WAY-181187, risperidone + WAY-181187, and olanzapine + WAY-181187; and the third consisted of 5 treatment groups: vehicle (1% Tween 80), Intepirdine (SB742457), haloperidol + SB-742457, risperidone + SB-742457, and olanzapine + SB-742457. One animal died during the administration of the compounds; therefore, one experimental group (i.e., risperidone + WAY-181187-treated group) eventually consisted of only 9 animals. 24 hours after the last drug administration, trained personnel sacrificed the rats by dislocating the cervical spinal cord.[2]
Haloperidol risperidone, olanzapine, WAY-181187 (oxalate), and Intepirdine (SB742457) were used in the experiment. Doses of APDs (haloperidol 0.5 mg/kg, risperidone 0.5 mg/kg, and olanzapine 5 mg/kg) and 5-HT6 ligands (WAY-181187 3 mg/kg and SB-742457 3 mg/kg) were selected for the experiments, based on literature review and our previous studies which presented their separate and combined behavioral effects. The compounds were suspended in a 1% solution of Tween 80 (Sigma Aldrich, St. Louis, MO, USA) immediately before administration and injected intraperitoneally (ip) in a volume of 2 mL/kg. The compounds were dispensed to the rats once daily between 10:00 and 11:00 a.m. for 28 days. The last injection was given 24 h before sacrifice. The control rats received 1% Tween 80, on the same dosing regimen.[2] The following drugs were used: WAY-181187 (oxalate), Intepirdine (SB742457), (+)-MK-801 (hydrogen maleate). All the compounds, except for MK-801 which was dissolved in distilled water, were suspended in 1% solution of Tween 80 immediately before administration, and were injected i.p. in a volume of 2 ml/kg. In acute experiments 5-HT6R ligands were injected 60 min before testing, while in chronic tests once a day during consecutive 21 days, with the last injection 24 h before the test. MK-801 was administered only once, 30 min before the tests. Control rats received vehicle according to the same schedule. The doses of drugs refer to their salt forms. [3] |
| 参考文献 |
|
| 其他信息 |
Intepirdine has been used in trials studying the treatment of Alzheimer's Disease. INTEPIRDINE is a small molecule drug with a maximum clinical trial phase of III (across all indications) and has 2 investigational indications.
In conclusion, the results obtained provide us with an unambiguous answer regarding whether the addition of a selective 5-HT6 agonist or antagonist will bring more benefits concerning post-APD metabolic disorders. The greatest benefits were obtained when the 5-HT6 ligand was co-administered with haloperidol, which, unlike risperidone (Ki = 420 nM) and olanzapine (Ki = 2.5 nM), has no affinity for the 5-HT6 receptor (Ki > 5000 nM). WAY-181187 normalized haloperidol-induced changes in the serum levels of peptides regulating appetite and metabolism activity and, to a lesser extent, decreased weight gain and food intake, while Intepirdine (SB742457) strongly reduced weight gain and food intake and was less likely to modify hormonal changes. Generally, SB-742457 more strongly inhibited increased weight gain and alleviated the hyperglycemia caused by APDs, but it should be noted that it also intensified dyslipidemia. On the other hand, WAY-181187 tended to improve the lipid profile, but increased the glucose level. It is also difficult to assess whether the modification of the serum levels of insulin, leptin, ghrelin, and adiponectin depended on the treatment applied or other drug-independent factors (for example: weight gain, daily locomotor activity, adipose tissue content); therefore, further research is needed.[2] MK-801 is a non-competitive antagonist of NMDA receptors (Wong et al., 1986) that induces cognitive disruptions similar to those associated with dementia (Ellison, 1995) and schizophrenia (Bubeníková-Valešová et al., 2008). MK-801-evoked memory deficit model is widely used in preclinical cognitive investigations (van der Staay et al., 2011). There are extensive number of animal tests sensitive to MK-801, including NORT and Y-CAT reflecting the rodent’s natural exploratory behaviors (Dix and Aggleton, 1999, Lalonde, 2002). In the present work, upon acute administration of MK-801, rats exhibited memory impairment when they were tested for their behavioral paradigms by NORT and Y-CAT, the tests reflecting episodic-like and spatial working memory processes, respectively. Then, the effects of acute and prolonged (21-day) i.p. administration of a selective 5-HT6R agonist, WAY-181187, and an antagonist, SB-745427, on these deficits induced by MK-801 were studied. In acute experiments, both, the 5-HT6R agonist (3 mg/kg) and the antagonist (1 and 3 mg/kg), prevented the cognitive impairments provoked by MK-801 in NORT. The memory enhancing effect of WAY-181187 was visibly stronger than that of Intepirdine (SB742457), but the difference of means did not reach the statistical significance (F(1, 13) = 1,9383, ns). Both 5-HT6R ligands, when given to animals once, also aided spatial memory task (Y-CAT), significantly improving the alternation performance of rats; WAY-181187 acted significantly at doses of 1 and 3 mg/kg while SB-742457 acted only at a dose of 3 mg/kg. Considering the efficacy of the two compounds given once at the same doses, slight differences can be observed in different memory function model. WAY-181187 showed a stronger effect in NORT at a higher dose than SB-742457 and a lack of activity when administered at a dose of 1 mg/kg. Moreover, DI value of WAY-181187, but not SB-742457, was clearly higher than that of control group, although statistically insignificant (one-way ANOVA followed by Bonferroni’s post hoc test revealed p = 0.08 vs vehicle-treated group). Such result suggests that some additional factors may be involved in the mechanism of pro-memory action of WAY-181187, but on this stage of studies the explanation of this phenomenon is difficult. In the Y-CAT model, the situation was reversed; pro-cognitive effects of both doses of WAY-181187 and a higher dose of SB-742457 were similar in efficacy and a lower dose of SB-742457 was inactive. In both tests, active doses of WAY-181187 and SB-742457 did not change exploratory activity of rats and so it can be assumed that the observed effects were specific. After chronic treatment with both selective 5-HT6R ligands, a significant improvement in MK-801-disrupted cognitive processes persisted, with a slightly stronger effect of WAY-181187 in NORT. But the favorable action of WAY-181187 and SB-742457 in Y-CAT was not observed any longer. At this stage of research, it is difficult to explain this loss of activity in Y-CAT after chronic administration of the two 5-HT6R ligands, while their beneficial effects in NORT persisted. The beneficial effect of 5-HT6R ligands on memory functions has been repeatedly reported in literature. More consistent results were obtained for 5-HT6R antagonists that were investigated in animal models of cognitive disorders. 5-HT6R antagonists were shown to be effective in paradigms of episodic (NORT) and spatial working memory (mazes or spontaneous alternation tasks), social cognition, and executive functions (set-shifting or reversal learning tasks) and in preventing memory impairments induced by scopolamine, phencyclidine (PCP), MK-801, ketamine, streptozotocin, as well as age-associated impairments (reviewed in Bokare et al. (2018); de Bruin and Kruse (2015); de Jong and Mørk (2017); Fone (2008); Upton et al. (2008)). However, there are only a few reports on SB-742457 activity in animal models of cognition. de Bruin et al. (2011) showed that Intepirdine (SB742457) ameliorated scopolamine-induced deficits in object recognition when administered i.p. acutely at doses of 3 and 10 mg/kg and at a dose of 10 mg/kg given per os (p.o.) it reduced scopolamine-induced deficits in object location task. The same authors reported that SB-742457 (0.63 mg/kg), when administered sub-chronically (for 5 days), attenuated PCP-induced deficits in reversal learning in a two-lever operant chamber task in rats (de Bruin et al., 2013); however, in a similar test performed by Idris et al., SB-742457 was active after acute subcutaneous (s.c.) administration at doses of 2.5 and 5 mg/kg (Idris et al., 2010). Callaghan et al. showed that a 7-day p.o. administration of 3 mg/kg of the compound reversed age-related deficits in middle-aged (13 months) rats in a delayed non-matching-to-sample task (Callaghan et al., 2012). Thus, the findings on SB-742457 activity presented in the present study match the available preclinical data, further enriching the knowledge of its pro-memory potential in rats, especially in a model of episodic memory.[3] |
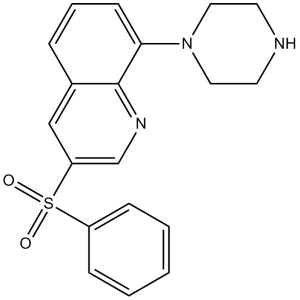
| 分子式 |
C19H19N3O2S
|
|
|---|---|---|
| 分子量 |
353.44
|
|
| 精确质量 |
353.119
|
|
| 元素分析 |
C, 64.57; H, 5.42; N, 11.89; O, 9.05; S, 9.07
|
|
| CAS号 |
607742-69-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11256720
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
608.3±45.0 °C at 760 mmHg
|
|
| 闪点 |
321.7±28.7 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.649
|
|
| LogP |
2.1
|
|
| tPSA |
70.68
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
535
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S(C1C([H])=C([H])C([H])=C([H])C=1[H])(C1=C([H])N=C2C(C([H])=C([H])C([H])=C2N2C([H])([H])C([H])([H])N([H])C([H])([H])C2([H])[H])=C1[H])(=O)=O
|
|
| InChi Key |
JJZFWROHYSMCMU-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2
|
|
| 化学名 |
3-(benzenesulfonyl)-8-piperazin-1-ylquinoline
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.07 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.07 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2% DMSO , 48% PEG300, 2% Tween 80 and 48% water: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8293 mL | 14.1467 mL | 28.2933 mL | |
| 5 mM | 0.5659 mL | 2.8293 mL | 5.6587 mL | |
| 10 mM | 0.2829 mL | 1.4147 mL | 2.8293 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A phase IIa/b double-blind, randomised, placebo-controlled, linear trend design dose-ranging study to investigate the effects of 24 weeks of monotherapy with SB-742457 on cognition in subjects with mild to moderate Alzheimer's disease
CTID: null
Phase: Phase 2 Status: Completed
Date: 2005-06-21
 |
|---|
 |
 |