| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
异佛尔酮可以选择性氧化为 4-羟基佛尔酮,这是一种颜料和药物化合物的合成中间体,也是一种重要的风味和香气成分。它是生物催化氧化的良好靶标[1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The demonstrated toxicity of isophorone by oral, inhalation, and dermal exposures indicates that it is capable of passage across epithelial membranes. Rabbits and rats treated orally with isophorone excreted unchanged isophorone in the expired air and in the urine. Preliminary results of a pharmacokinetic study indicate that rats treated orally with 14C-isophorone excreted 93% of the radiolabel in the urine, expired air & feces in 24 hr. The majority was found in the urine indicating that isophorone was well absorbed. The wide distribution of isophorone in the organs of rats & a rabbit 1-5 hr after dosing by gavage with 4000 mg/kg indicates rapid GI absorption. In two rabbits given a gavage dose of 1000 mg/kg isophorone, a blood level of isophorone of 102 ug/L was found within 10 min. The level increased to 141 ug/L in 30 min & declined to < or = 0.05 ug/L in 21 hr. The results indicate rapid absorption & elimination. The detection of unchanged isophorone & its metabolites in the urine & the observations of systemic toxicity & carcinogenicity in animals exposed orally to isophorone provide qualitative evidence that isophorone is absorbed after oral exposure. In rats exposed to 400 ppm isophorone for 4 hr & sacrificed immediately after exposure or 1.5 or 3 hr after exposure, levels of isophorone were highest in all tissues examined (brain, lungs, heart, stomach, liver, spleen, pancreas, kidney, adrenals, testicles, & ovaries) immediately after exposure. Levels ranged from 1.5-74 ug/g tissue wet weight. The levels declined rapidly in males but declined very little in females by 3 hr after exposure. Radiolabel was widely distributed in male rats 24 hr after an oral dose of 14C-isophorone in corn oil, with highest levels in the liver, kidney, preputial gland, testes, brain, & lungs. Isophorone was widely distributed to the tissues of rats & a rabbit following treatment with isophorone at a gavage dose of 4000 mg/kg. The rats died within 1-5 hr & the rabbit died within an hr after dosing at which times the tissues were sampled for analysis. in rats, tissue levels of isophorone in ug/g tissue wet weight were as follows: stomach-6213, pancreas-2388, adrenals-1513, spleen-1038, liver-613, brain-378, lung-383, heart-387, kidney-465, testes-275, & ovaries-471. In the rabbit, tissue levels were as follows: stomach-5395, adrenals-1145, ovaries-3000, spleen-545, liver-515, kidney-295, heart-260, & lungs-50. Metabolism / Metabolites ... ISOPHORONE IS METABOLIZED IN RABBITS INTO 5,5-DIMETHYLCYCLOHEX-1-EN-3-ONE-1-CARBOXYLIC ACID, WHICH IS EXCRETED IN URINE AS ESTER GLUCURONIDE. AFTER ORAL ADMIN OF 1 G/KG ALPHA-ISOPHORONE, RABBIT & RAT URINE CONTAINED ALPHA-ISOPHORONE, ISOPHOROL, CIS-3,5,5-TRIMETHYLCYCLOHEXANOL, TRANS-3,5,5-TRIMETHYLCYCLOHEXANOL, DIHYDROISOPHORONE, 5,5-DIMETHYLCYCLOHEX-1-EN-3-ONE-1-CARBOXYLIC ACID, & DIHYDROISOPHORONE GLUCURONIDE. The allylic methyl group of isophorone was oxidized to a carboxylic acid group when industrial isophorone was administered orally to rabbits. The product was detected in urine and no other products were identified. Rabbits & rats treated orally with isophorone excreted unchanged isophorone in the expired air & in the urine. The urine also contained 3-carboxy-5,5-dimethyl-2-cyclohexene-1-one & glucuronic conjugates of 3,3,5-trimethyl-2-cyclohexene-1-ol (isophorol), 3,5,5,-trimethylcyclohexanone (dihydroisophorone), & cis- & trans-3,5,5-trimethylcyclohexanols. Rat urine contained more dihydroisophorone & less isophorol than did rabbit urine. ... /It was/ proposed that metab of isophorone involves methyloxidation to 3-carboxy-5,5-dimethyl-2-cyclohexene-1-one, reduction of the ketone group to isophorol, reduction of the ring double bond to dihydroisophorone, & dismutation of dihydroisophorone to cis- & trans-3,5,5-trimethylcyclohexanols. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Isophorone is a colorless liquid with a peppermint like odor. It is soluble in water and miscible with most organic solvents. HUMAN EXPOSURE: The odor of isophorone can be detected at low concentrations. Eye, nose and throat irritation has been observed along with nausea, headache, dizziness, faintness and inebriation. Dermal and inhalation exposure may occur along with oral exposure from drinking water. ANIMAL STUDIES: Distribution studies in rats using (14)C isophorone showed that 93% of orally administered radioactivity appeared mainly in the urine and expired air within 24 hr. The tissues retaining the highest concentration after this period were the liver, kidney and preputial glands. The metabolites from oral administration of isophorone identified in rabbit urine resulted from the oxidation of the 3-methyl group, reduction of the keto group and hydrogenation of the double bond of the cyclohexene ring, and were eliminated as such or as glucuronide derivatives in the case of the alcohols. In animal studies, data indicate that isophorone is rapidly absorbed through the skin. Acute effects from dermal exposure in rats and rabbits ranged from mild erythema to scabs. Conjunctivitis and corneal damage have been reported following direct application to the eye or exposure to high concentrations of isophorone. In acute and short-term oral studies on rodents at high doses degenerative effects were seen in the liver and CNS depression and some deaths. In a 90 day oral study in beagle dogs (with limited numbers) no effects were seen at doses up to 150 mg/kg body weight per day. Isophorone does not induce gene mutations in bacteria, chromosomal aberrations in vitro, DNA repair in primary rat hepatocytes, or bone marrow micronuclei in mice. Positive effects were observed only in the absence of an exogenous metabolic system in L5178YTK +/- mouse mutagenesis assays as well as in a sister chromatid exchange assay. Isophorone induced morphological transformation in vitro in the absence of an exogenous metabolism system. It did not induce sex linked recessive lethal mutations in Drosophilia. In long term oral toxicity studies in mice and rats, male rats showed several lesions of the kidney, including nephropathy, tubular cell hyperplasia and low incidence of tubular cell adenomas and adenocarcinomas. Isophorone exposure was associated with some neoplastic lesions of the liver, and the integumentary and lymphoreticular systems of male mice, as well non-neoplastic liver and adrenal cortex lesions, but this was not observed in female mice. In /one/ long term inhalation study in rats and rabbits, irritation to eye and nasal mucosa, and lung and liver changes were observed. Very limited studies in rats and mice indicate that isophorone does not affect fertility nor does it cause developmental toxicity in experimental animals. The fact that central nervous system depression occurs in experimental animals could indicate a positive neurotoxic effect. Isophorone also elicited a positive effect in the behavioral despair swimming test. No data on terrestrial animals were available. The available data suggest that isophorone has a low toxicity to aquatic organisms. Toxicity Data LC50 (rat) = 7,000 mg/m3/4h Interactions Joint toxic action of isophorone with 26 industrial liquid chemicals was examined based on acute LD50 data from oral intubations of female albino rats. ... LD50s were determined for each of the cmpds. Based on the assumption of simple similar action, isophorone exhibited >additive toxicity in combination with 9 cmpds & ... /It was/ reported that inhalation of isophorone for 4 hr by mice increased the threshold for onset of seizures produced by iv admin of pentrazole ... . Non-Human Toxicity Values LD50 Rat oral 1000-3450 mg/kg. LC50 Rabbit dermal 1380 mg/kg LC50 Rat inhalation 7000 mg/cu m/4 hr LD50 Mouse oral 2.0 g/kg For more Non-Human Toxicity Values (Complete) data for ISOPHORONE (10 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Isophorone is a clear liquid that smells like peppermint. It can be dissolved in water and evaporates somewhat faster than water. It is an industrial chemical used as a solvent in some printing inks, paints, lacquers, and adhesives. It is also used as an intermediate in the production of certain chemicals. Although isophorone is an industrial chemical, it also occurs naturally in cranberries.
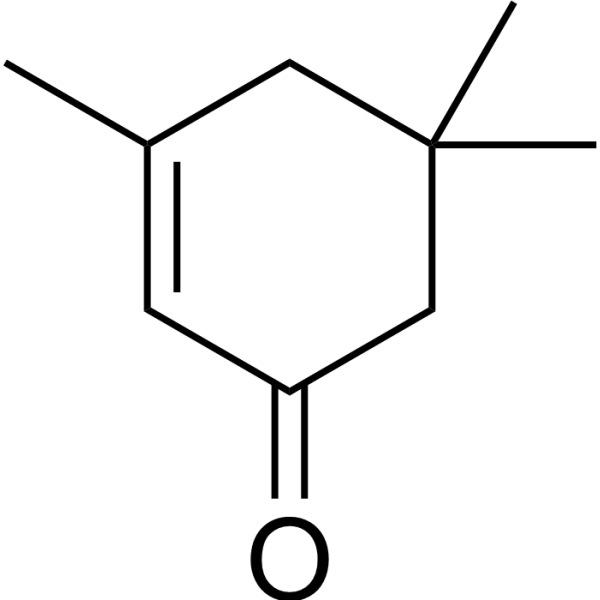
Isophorone appears as a clear colorless liquid, with a camphor-like odor. Less dense than water and insoluble in water. Boiling point 420 °F. Flash point near 200 °F. Contact irritates skin and eyes. Toxic by ingestion. Used as a solvent and in pesticides. Isophorone is a cyclic ketone, the structure of which is that of cyclohex-2-en-1-one substituted by methyl groups at positions 3, 5 and 5. It has a role as a solvent and a plant metabolite. It is a cyclic ketone and an enone. Isophorone is a widely used solvent and chemical intermediate. The acute (short-term) effects of isophorone in humans from inhalation exposure include eye, nose, and throat irritation. Chronic (long- term) exposure to isophorone in humans can cause dizziness, fatigue, and depression. Animal studies indicate that long-term inhalation of high concentrations of isophorone causes central nervous system effects. Limited evidence in animal studies suggests that isophorone may cause birth defects such as fetal malformations and growth retardation from inhalation exposure to isophorone during pregnancy. No information is available on the reproductive, developmental, or carcinogenic effects of isophorone in humans. EPA has classified isophorone as a Group C, possible human carcinogen. Isophorone has been reported in Artemisia judaica, Vaccinium macrocarpon, and other organisms with data available. |
| 分子式 |
C9H14O
|
|---|---|
| 分子量 |
138.21
|
| 精确质量 |
138.104
|
| CAS号 |
78-59-1
|
| 相关CAS号 |
Isophorone-d5;1262769-87-8;Isophorone-d8;14397-59-2
|
| PubChem CID |
6544
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
0.9±0.1 g/cm3
|
| 沸点 |
215.2±0.0 °C at 760 mmHg
|
| 熔点 |
−8 °C(lit.)
|
| 闪点 |
84.4±0.0 °C
|
| 蒸汽压 |
0.2±0.4 mmHg at 25°C
|
| 折射率 |
1.455
|
| LogP |
2.07
|
| tPSA |
17.07
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
187
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C([H])=C(C([H])([H])[H])C([H])([H])C(C([H])([H])[H])(C([H])([H])[H])C1([H])[H]
|
| InChi Key |
HJOVHMDZYOCNQW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H14O/c1-7-4-8(10)6-9(2,3)5-7/h4H,5-6H2,1-3H3
|
| 化学名 |
3,5,5-trimethylcyclohex-2-en-1-one
|
| 别名 |
NSC-403657; NSC 403657; Isophorone
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~723.54 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (18.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (18.09 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (18.09 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.2354 mL | 36.1768 mL | 72.3537 mL | |
| 5 mM | 1.4471 mL | 7.2354 mL | 14.4707 mL | |
| 10 mM | 0.7235 mL | 3.6177 mL | 7.2354 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。