| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
ERRα; ERRγ; Topo I; fatty acid synthase
|
|---|---|
| 体外研究 (In Vitro) |
Kaempferol 还通过下调 NFκB 通路和抑制 Src 激酶来抑制环加氧酶 2 和白细胞介素 4 的表达。这会产生抗炎作用。此外,山奈酚可以很好地阻止血管生成并导致卵巢癌细胞死亡。山奈酚是一种在许多水果和蔬菜中发现的天然类黄酮,长期研究表明,几十年来,它可以显着降低从事护理工作的美国女护士患卵巢癌的风险。 24 小时治疗后,山奈酚显着且浓度依赖性地抑制所有三种测试的卵巢癌细胞的增殖。在 40 μM 或更高的处理浓度下,可以看到这种抑制作用。山奈酚是一种黄酮类化合物,广泛存在于许多由传统医学中使用的植物和叶子制成的食品中。值得注意的是,山奈酚抑制 NADPH 氧化酶的活性。山奈酚通过直接结合 NADPH 氧化酶来减少活性氧 (ROS)。山奈酚抑制 CAMKII 氧化,从而阻止 Ang II 诱导窦房结细胞死亡。 10-20 μM 的药物可剂量依赖性地抑制致敏 RBL-2H3 细胞中山奈酚的释放。当添加 10–20 μM 山奈酚 15 分钟时,DNP-BSA 攻击的 RBL-2H3 细胞中 Syk 和 PLCγ 的激活大大降低。当添加 ≥10 μM 山奈酚 60 分钟时,DNP-BSA 攻击的 RBL-2H3 细胞中 COX2 的诱导作用会减弱。
|
| 体内研究 (In Vivo) |
接受 BSA 攻击的 BALB/c 小鼠已证实其气道中存在 COX2 诱导。未经治疗的对照小鼠的气道被发现缺乏COX2。当小鼠口服 BSA 时,它们的气道更容易诱导 COX2(深棕色染色),这也可以通过口服山奈酚来抵消。在给予 BSA 的小鼠中观察到上皮增厚和杯状细胞明显增生。在给予 BSA 的小鼠中,当给予 20 mg/kg 山奈酚补充剂时,上皮增厚完全消失。
|
| 酶活实验 |
将右心房或窦房结细胞在裂解缓冲液(50 mM Tris-HCl pH 7.5、100 mM KCl、1 mM 乙二胺四乙酸、1 mM 乙二醇四乙酸、1 mM 二硫苏糖醇、0.1 mM 苯甲基磺酰氟、0.5 mM 苯甲脒)中均质化、20 mg/L 亮肽素、20 mM 焦磷酸钠、50 mM NaF 和 50 mM β-甘油磷酸钠)。 Bradford 测定用于量化总蛋白质含量。 EnzChek 的 Caspase-3 检测试剂盒用于测量 caspase-3 活性[3]。
|
| 细胞实验 |
将卵巢癌细胞以 2000 个细胞/孔的密度接种到 96 孔板中并孵育过夜后,进行三重选择的 0-160 μM 山奈酚治疗 24 小时。除去培养基后,通过冷冻和解冻平板来裂解细胞。向每孔中添加 200 μL 含有 5 倍 SYBR Green I 的 1× CyQUANT 细胞裂解缓冲液,然后在室温 (RT) 下孵育 5 分钟。将反应液 (50 µL) 转移至 PCR 带管后,Chromo4 PCR 装置会在 90°C 下实时测量荧光信号。过夜孵育后测量基因组 DNA 丰度,并通过在 96 孔板中接种不同数量的 OVCAR-3 细胞(基于血细胞计数器计数)来创建标准曲线。这确保了细胞增殖测定在细胞数量的线性范围内进行。将来自三个独立实验的数据合并起来进行统计分析[2]。
|
| 动物实验 |
Mice: The four treatment groups (n=8 per group) are randomly assigned to three-week-old male BALB/c mice. (1) PBS-sensitized mice; (2) BSA-sensitized mice; (3) BSA-sensitized and 10 mg/kg Kaempferol-administered mice; and (4) BSA-sensitized and 20 mg/kg Kaempferol-administered mice. A commercial mouse chow diet consisting of 20.5% protein, 3.5% fat, 8% fiber, 8% ash, and 0.5% phosphorus is fed to the mice, and they are given unlimited access to food and water. The mice are housed in particular pathogen-free conditions with a 12-hour light and dark cycle, 23±1°C, and 50%±5% relative humidity. Prior to beginning the allergy experiments, mice are given a week to acclimate to their new environment. All experimental mice are sensitized by subcutaneous injection on days 0 and 14 with 20 μg BSA in 30 μL PBS and 50 μL inject alum. A combination of 50 μL PBS and 50 μL Imject Alum without BSA is injected into the control mice. Days 28, 29, and 30 involve giving 5% BSA inhalation only to the experimental mice that have become sensitized to it; control mice are given 5% PBS for 20 minutes in a plastic chamber that is connected to a Medel aerosol nebulizer. A full day after the final challenge, all mice are killed. Neutrophils, basophils, and eosinophils are directly counted from whole blood samples. Before being used, the right lung is kept in 4% paraformaldehyde.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The aim of this study was to assess kaempferol bioavailability in healthy humans, after bean (Phaseolus vulgaris L.) consumption through the monitoring of the excretion in relation to intake. In seven healthy subjects receiving kaempferol from cooked bean, maximum excretion of hydrolyzed flavonol was obtained after 2-8 hr. Intersexual variations in urinary excretion were found to be 6.10+/-5.50% and 5.40+/-5.40% of the kaempferol dose for male and female subjects, respectively. Although a 6.72-fold inter-individual variation between the highest and lowest excretion concentrations was found, all individuals exhibited similar excretion profiles. Moreover, a direct correlation between the percentage of kaempferol excreted and the body mass index of volunteers was observed with a correlation index equal to 0.80. All except two individuals exhibited a first peak of kaempferol excretion 2 hr after ingestion. The study reveals information about inter-individual excretion capacity after kaempferol intake and that kaempferol can be used as a biomarker for flavonol consumption. ... A pharmacokinetic study of kaempferol from endive ... /was studied in / four healthy males and four healthy females. Kaempferol, from a relatively low dose (9 mg), was absorbed from endive with a mean maximum plasma concentration of 0.1 uM, at a time of 5.8 hr, indicating absorption from the distal section of the small intestine and/or the colon. Although a 7.5-fold interindividual variation between the highest and lowest maximum plasma concentration was observed, most individuals showed remarkably consistent pharmacokinetic profiles. This contrasts with profiles for other flavonoids that are absorbed predominantly from the large intestine (eg rutin). An average of 1.9% of the kaempferol dose was excreted in 24 hr. Most subjects also showed an early absorption peak, probably corresponding to kaempferol-3-glucoside, present at a level of 14% in the endive. Kaempferol-3-glucuronide was the major compound detected in plasma and urine. Quercetin was not detected in plasma or urine indicating a lack of phase I hydroxylation of kaempferol. Kaempferol is absorbed more efficiently than quercetin in humans even at low oral doses. The predominant form in plasma is a 3-glucuronide conjugate, and interindividual variation in absorption and excretion is low, suggesting that urinary kaempferol could be used as a biomarker for exposure. Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. Quercetin and kaempferol in different period of human urine were determined by using RP-HPLC. The results showed the elimination rate constant k and the absorption rate constant ka of quercetin were slightly more than that of kaempferol; and the absorption half-life (t(1/2a)), the elimination half-life (t(1/2)) and t(max) of quercetin were less than that of kaempferol, the differences were, however, not statistically significant. The mean values of ka were 0.61 hr(-1) and 0.55 hr(-1), t(1/2a) 1.51 hr and 1.56 hr, k 0.37 hr(-1) and 0.30 hr(-1), t(1/2) 2.17 hr and 2.76 hr, T(max) 2.30 hr and 2.68 hr for quercetin and kaempferol, respectively, which mean absorption and elimination of quercetin and kaempferol are 0.17% and 0.22%, respectively. Quercetin and kaempferol are excreted in the human urine mainly as glucuronides. The objective of this study was to investigate whether kaempferol and quercetin could be transported into primary cultured cerebral neurons, to establish a practical HPLC method with UV detection for the two flavonols in the neurons, and to study the uptake and transport behaviors of them through the neurons. The present results showed that the level of kaempferol in the neurons increased linearly and then reached a plateau with incubation time at the high concentration of 10 ?g/mL, but not at the other two concentrations of 1 and 0.1 ug/mL. However, the levels of quercetin in the neurons were not detected at the three incubating concentrations, and there was a new peak detected in the cell whose retention time was shorter (3.42 min) than that of quercetin (4.65 min). This phenomenon suggested that quercetin might be transported into the neurons and then metabolized quickly to some derivative. Kaempferol could be transported into the neurons in a concentration- and time-dependent manner when the neurons were incubated with the culture medium containing kaempferol at the high dose. There was an apparent correlation between the concentrations of kaempferol in the medium and in the cell, indicating that the uptake of kaempferol in the cell increased along with its dose (10 ug/mL). However, there was a negative correlation between the concentrations of quercetin in the medium and in the cell. The results suggested that kaempferol and quercetin were disposed by the neurons at different way, and this might be an important factor for their different effects on primary cultured cortical cells. Metabolism / Metabolites To elucidate the metabolism of hispidulin in the large intestine, its biotransformation by the pig caecal microflora was studied. In addition, the efficiency of the pig caecal microflora to degrade galangin (3,5,7-trihydroxyflavone), kaempferol (3,5,7,4?-tetrahydroxyflavone), apigenin (5,7,4?-trihydroxyflavone), and luteolin (5,7,3?,4?- tetrahydroxyflavone) was investigated. Identification of the formed metabolites was performed by high-performance liquid chromatography (HPLC)-diode array detection, HPLC-electrospray ionization-tandem mass spectrometry, and high-resolution gas chromatography-mass spectrometry. The caecal microflora transformed ... kaempferol to 4-hydroxyphenylacetic acid, phloroglucinol, and 4-methylphenol; ... To elucidate to what extent different hydroxylation patterns on the B-ring influence the degradation degree of flavonoids, the conversions of galangin and kaempferol as well as that of apigenin and luteolin were compared with those of quercetin (3,5,7,3?, 4?-pentahydroxyflavone) and chrysin (5.7-dihydroxyflavone), respectively. Regardless of the flavonoid subclass, the presence of a hydroxy group at the 4?-position seems to be a prerequisite for fast breakdown. An additional hydroxy group at the B-ring did not affect the degradation degree. The metabolism of the flavonoids quercetin and kaempferol by rat hepatocytes was investigated using liquid chromatography coupled with electrospray mass spectrometry (LC-ESI MS). Quercetin and kaempferol were extensively metabolized (98.8 +/- 0.1% and 81.0 +/- 5.1% respectively, n = 4), with four glucuronides of quercetin and two of kaempferol being detected after incubation. The glucuronides of quercetin and kaempferol formed upon incubation with rat hepatocytes were identified as the same ones formed after incubation with the UDP-glucuronosyltransferase isoform UGT1A9. In addition, plasma samples from human volunteers taken after consumption of capsules of Ginkgo biloba, a plant rich in flavonoid glycosides, were analysed by LC-MS for the presence of flavonoid glucuronides and flavonoid glycosides. Reported is evidence for the presence of flavonoid glycosides in samples of plasma. The results suggest that UGT1A9 is a key UDP-glucuronosyltransferase isoform for the metabolism of flavonoids, and that absorption of intact flavonoid glycosides is possible. Kaempferol is a flavonoid widely distributed in edible plants and has been shown to be genotoxic to V79 cells in the absence of external metabolizing systems. The presence of an external metabolizing system, such as rat liver homogenates (S9 mix), leads to an increase in its genotoxicity, which is attributed to its biotransformation to the more genotoxic flavonoid quercetin, via the cytochrome P450 (CYP) mono-oxygenase system. ... Kaempferol has known human metabolites that include Kaempferol-3-glucuronide and (2S,3S,4S,5R)-6-[3,5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid. Kaempferol is a known human metabolite of galangin and kaempferide. Biological Half-Life Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. ... The absorption half life was 1.51 hr and elimination half-life was 1.56 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
It has been reported that tamoxifen is a substrate of P-glycoprotein (P-gp) and microsomal cytochrome P450 (CYP) 3A, and kaempferol is an inhibitor of P-gp and CYP3A. Hence, it could be expected that kaempferol would affect the pharmacokinetics of tamoxifen. Thus, tamoxifen was administered orally (10 mg/kg) without or with oral kaempferol (2.5 and 10 mg/kg). In the presence of kaempferol, the total area under the plasma concentration-time curve from time zero to time infinity (AUC) of tamoxifen was significantly greater, C(max) was significantly higher and F was considerably greater than those without kaempferol. The enhanced bioavailability of oral tamoxifen by oral kaempferol could have been due to an inhibition of CYP3A and P-gp by kaempferol. The presence of kaempferol did not alter the pharmacokinetic parameters of a metabolite of tamoxifen, 4-hydroxytamoxifen. This could have been because the contribution of CYP3A to the formation of 4-hydroxytamoxifen is not considerable in rats. This study was to investigate the effect of kaempferol on the pharmacokinetics of etoposide after oral or intravenous administration of etoposide in rats. The oral (6 mg/kg) or intravenous (2 mg/kg) etoposide was administered to rats alone or 30 min after the oral kaempferol (1, 4, or 12 mg/kg) administration. Compared to the oral control group, the presence of kaempferol significantly (4 mg/kg, P < 0.05; 12 mg/kg, P < 0.01) increased the area under the plasma concentration time curve (AUC) and the peak concentration (C(max)) of the oral etoposide. Kaempferol decreased significantly (4 or 12 mg/kg, P < 0.05) the total body clearance (CL/F) of oral etoposide, while there was no significant change in the terminal halflife (t(1/2)), the elimination rate constant (K(el)) and the time to reach the peak concentration (T(max)) of etoposide in the presense of kaempferol. Consequently, the absolute bioavailability (AB%) of oral etoposide with kaempferol was significantly higher (4 mg/kg, P < 0.05; 12 mg/kg, P < 0.01) than those from the control group. Compared to the intravenous control group, the presence of kaempferol enhanced the AUC of intravenously administered etoposide, however, only presence of 12 mg/kg of kaempferol significant (P < 0.05) increased AUC of etoposide. The enhanced bioavailability of oral etoposide by kaempferol could have been due to an inhibition of cytochrom P450 (CYP) 3A and P-glycoprotein (P-gp) in the intestinal or decreased total body clearance in the liver by kaempferol. The dosage regimen of etoposide should be taken into consideration for potential drug interaction when combined with kaempferol or dietary supplements containing kaempferol in patients. Twenty male SD rats, weighing 220-260 g, were distributed randomly into 4 groups. The animals were fasted, but allowed free access to water for 12 hr before the administration of drugs. Nifedipine dissolved in corn oil was administered via gastric intubation to the rats in control group at a dose of 10 mg/kg. Kaempferol was administered orally to the other three groups with dose of 5, 10, 15 mg/kg, respectively, followed by oral administration of nifedipine 10 mg/kg. Blood samples were collected through tail vein in heparinized plastic microcentrifuge tubes before and after drug administration. The plasma concentration of NFP was monitored with reversed phase high-performance liquid chromatography (RP-HPLC). Nimodipine was used as the internal standard. Statistical data evaluation was performed with Student's t-test and one-way analysis of variances. The maximal plasma concentration (C(max)) of the three treated groups were 0.51, 0.70 and 0.81 microg/ml, respectively. The area under the concentration-time curve (AUC(0-8)) were 1.81, 2.83 and 3.63 ug/(hr.mL(-1)), respectively. The C(max), AUC(0-8) and the mean retention time (MRT(0-8)) of nifedipine were significantly increased by simultaneous oral treatment with kaempferol (P<0.01). On the other hand, there were no significant differences in the mean peak value time in plasma (T(max)) and the elimination half-life (t1/2(ke)) between the control and the treated groups. The concomitant oral use of kaempferol with nifedipine may influence the pharmacokinetic parameters of nifedipine in rats, which suggests that kaempferol might reduce the first-pass metabolism of nifedipine. Quercetin, kaempferol and biapigenin significantly reduced neuronal death caused by 100 uM kainate plus 100 uM N-methyl-D-aspartate. The observed neuroprotection was correlated with prevention of delayed calcium deregulation and with the maintenance of mitochondrial transmembrane electric potential. The three compounds were able to reduce mitochondrial lipid peroxidation and loss of mitochondrial transmembrane electric potential caused by oxidative stress induced by ADP plus iron. ... the results suggest that the neuroprotective action induced by quercetin and kaempferol are mainly mediated by antioxidant effects ... |
| 参考文献 |
|
| 其他信息 |
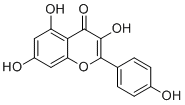
Kaempferol is a tetrahydroxyflavone in which the four hydroxy groups are located at positions 3, 5, 7 and 4'. Acting as an antioxidant by reducing oxidative stress, it is currently under consideration as a possible cancer treatment. It has a role as an antibacterial agent, a plant metabolite, a human xenobiotic metabolite, a human urinary metabolite, a human blood serum metabolite and a geroprotector. It is a member of flavonols, a 7-hydroxyflavonol and a tetrahydroxyflavone. It is a conjugate acid of a kaempferol oxoanion.
Kaempferol has been reported in Hydrangea serrata, Caragana frutex, and other organisms with data available. Kaempferol is a natural flavonoid which has been isolated from Delphinium, Witch-hazel, grapefruit, and other plant sources. Kaempferol is a yellow crystalline solid with a melting point of 276-278 degree centigrade. It is slightly soluble in water, and well soluble in hot ethanol and diethyl ether. Kaempferol is a metabolite found in or produced by Saccharomyces cerevisiae. See also: Cannabis sativa subsp. indica top (part of); Tussilago farfara flower (part of). Mechanism of Action Pure kaempferol and a number of related flavonoids were examined as MAOIs in-vitro. Kaempferol, apigenin and chrysin proved to be potent monoamine oxidase (MAO) inhibitors (MAOI)s, but produced more pronounced inhibition of MAO-A than MAO-B. IC50 (50% inhibition concentration) values for the ability of these three flavones to inhibit MAO-A were 7 x 10(-7), 1 x 10(-6) and 2 x 10(-6) M, respectively. Ginkgo biloba leaf extract and kaempferol were found to have no effect ex-vivo on rat or mouse brain MAO or on concentrations of dopamine, noradrenaline, 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Kaempferol was shown to protect against N-methyl-D-aspartate-induced neuronal toxicity in-vitro in rat cortical cultures, but did not prevent DSP-4-induced noradrenergic neurotoxicity in an in-vivo model. Both Ginkgo biloba extract and kaempferol were demonstrated to be antioxidants in a lipid-peroxidation assay. This data indicates that the MAO-inhibiting activity of Ginkgo biloba extract is primarily due to the presence of kaempferol. Ginkgo biloba extract has properties indicative of potential neuroprotective ability. Kaempferol is a dietary flavonoid that is thought to function as a selective estrogen receptor modulator. ... This study ... established that kaempferol also functions as an inverse agonist for estrogen-related receptors alpha and gamma (ERRalpha and ERRgamma). ... Kaempferol binds to ERRalpha and ERRgamma and blocks their interaction with coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha). Kaempferol also suppressed the expressions of ERR-target genes pyruvate dehydrogenase kinase 2 and 4 (PDK2 and PDK4). This evidence suggests that kaempferol may exert some of its biological effect through both estrogen receptors and estrogen-related receptors. Therapeutic Uses /EXPL/ Despite recent advances in understanding molecular mechanisms involved in glioblastoma progression, the prognosis of the most malignant brain tumor continues to be dismal. Because the flavonoid kaempferol is known to suppress growth of a number of human malignancies, we investigated the effect of kaempferol on human glioblastoma cells. Kaempferol induced apoptosis in glioma cells by elevating intracellular oxidative stress. Heightened oxidative stress was characterized by an increased generation of reactive oxygen species (ROS) accompanied by a decrease in oxidant-scavenging agents such as superoxide dismutase (SOD-1) and thioredoxin (TRX-1). Knockdown of SOD-1 and TRX-1 expression by small interfering RNA (siRNA) increased ROS generation and sensitivity of glioma cells to kaempferol-induced apoptosis. Signs of apoptosis included decreased expression of Bcl-2 and altered mitochondrial membrane potential with elevated active caspase-3 and cleaved poly(ADP-ribose) polymerase expression. Plasma membrane potential and membrane fluidity were altered in kaempferol-treated cells. Kaempferol suppressed the expression of proinflammatory cytokine interleukin-6 and chemokines interleukin-8, monocyte chemoattractant protein-1, and regulated on activation, normal T-cell expressed and secreted. Kaempferol inhibited glioma cell migration in a ROS-dependent manner. Importantly, kaempferol potentiated the toxic effect of chemotherapeutic agent doxorubicin by amplifying ROS toxicity and decreasing the efflux of doxorubicin. Because the toxic effect of both kaempferol and doxorubicin was amplified when used in combination, this study raises the possibility of combinatorial therapy whose basis constitutes enhancing redox perturbation as a strategy to kill glioma cells. /EXPL/ Kaempferol is one of the most important constituents in ginkgo flavonoids. Recent studies indicate kaempferol may have antitumor activities. The objective of this study was to determine the effect and mechanisms of kaempferol on pancreatic cancer cell proliferation and apoptosis. Pancreatic cancer cell lines MIA PaCa-2 and Panc-1 were treated with kaempferol, and the inhibitory effects of kaempferol on pancreatic cancer cell proliferation were examined by direct cell counting, 3H-thymidine incorporation, and MTS assay. Lactate dehydrogenase release from cells was determined as an index of cytotoxicity. Apoptosis was analyzed by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling assay. Upon the treatment with 70 microm kaempferol for 4 days, MIA PaCa-2 cell proliferation was significantly inhibited by 79% and 45.7% as determined by direct cell counting and MTS assay, respectively, compared with control cells (P < 0.05). Similarly, the treatment with kaempferol significantly inhibited Panc-1 cell proliferation. Kaempferol treatment also significantly reduced 3H-thymidine incorporation in both MIA PaCa-2 and Panc-1 cells. Combination treatment of low concentrations of kaempferol and 5-fluorouracil showed an additive effect on the inhibition of MIA PaCa-2 cell proliferation. Furthermore, kaempferol had significantly less cytotoxicity than 5-fluorouracil in normal human pancreatic ductal epithelial cells (P = 0.029). In both MIA PaCa-2 and Panc-1 cells, apoptotic cell population was increased when treated with kaempferol in a concentration-dependent manner. CONCLUSIONS: Ginkgo biloba extract kaempferol effectively inhibits pancreatic cancer cell proliferation and induces cancer cell apoptosis, which may sensitize pancreatic tumor cells to chemotherapy. Kaempferol may have clinical applications as adjuvant therapy in the treatment of pancreatic cancer. /EXPL/ Dietary flavonols have been found to possess preventive and therapeutic potential against several kinds of cancers. This study is conducted to investigate the anti-proliferation effects of kaempferol, a major component of food flavonols, against colon cancer cells. In the human HCT116 colon cancer cell line, kaempferol induced p53-dependent growth inhibition and apoptosis. Furthermore, kaempferol was found to induce cytochrome c release from mitochondria and activate caspase-3 cleavage. The Bcl-2 family proteins including PUMA were involved in this process. Kaempferol also induced ATM and H2AX phosphorylation in HCT116 cells, inhibition of ATM by a chemical inhibitor resulted in abrogation of the downstream apoptotic cascades. These findings suggest kaempferol could be a potent candidate for colorectal cancer management. /EXPL/ ... Treatment of the chronic myelogenous leukemia cell line K562 and promyelocitic human leukemia U937 with 50 microM kaempferol resulted in an increase of the antioxidant enzymes Mn and Cu/Zn superoxide dismutase (SOD). Kaempferol treatment induced apoptosis by decreasing the expression of Bcl-2 and increasing the expressions of Bax. There were also induction of mitochondrial release of cytochrome c into cytosol and significant activation of caspase-3, and -9 with PARP cleavage. Kaempferol treatment increased the expression and the mitochondria localization of the NAD-dependent deacetylase SIRT3. K562 cells stably overexpressing SIRT3 were more sensitive to kaempferol, whereas SIRT3 silencing did not increase the resistance of K562 cells to kaempferol. Inhibition of PI3K and de-phosphorylation of Akt at Ser473 and Thr308 was also observed after treating both K562 and U937 cells with kaempferol. ... Oxidative stress induced by kaempferol in K562 and U937 cell lines causes the inactivation of Akt and the activation of the mitochondrial phase of the apoptotic program with an increase of Bax and SIRT3, decrease of Bcl-2, release of cytochrome c, caspase-3 activation, and cell death. /EXPL/ Atherosclerosis is a chronic inflammatory disease of the arterial wall. Kaempferol and rhamnocitrin (kaempferol 7-O-methyl ether) are two anti-inflammatory flavonoids commonly found in plants. The aim of this study is to investigate the function of kaempferol and rhamnocitrin on prevention of atherosclerosis. Chemical analyses demonstrated that kaempferol and rhamnocitrin were scavengers of DPPH (1,1-diphenyl-2-picrylhydrazyl) with IC50 of 26.10 +/- 1.33 and 28.38 +/- 3.07 microM, respectively. Copper-induced low-density lipoprotein (LDL) oxidation was inhibited by kaempferol and rhamnocitrin, with similar potency, as measured by decreased formation of malondialdehyde and relative electrophoretic mobility (REM) on agarose gel, while rhamnocitrin reduced delayed formation of conjugated dienes better than kaempferol. Cholesterol-laden macrophages are the hallmark of atherogenesis. The class B scavenger receptor, CD36, binds oxidized low-density lipoprotein (oxLDL), is found in atherosclerotic lesions, and is up-regulated by oxLDL. Addition of kaempferol and rhamnocitrin (20 microM) caused significant reductions in cell surface CD36 protein expression in THP-1-derived macrophages (p < 0.05). Reverse transcription quantitative PCR (RT-Q-PCR) showed that kaempferol and rhamnocitrin (20 microM) decreased oxLDL-induced CD36 mRNA expression (p < 0.01 and p < 0.05, respectively). Kaempferol- and rhamnocitrin-treated macrophages also showed reduction in 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanide perchlorate (DiI)-labeled oxLDL uptake. Current evidences indicate that kaempferol and rhamnocitrin not only protect LDL from oxidation but also prevent atherogenesis through suppressing macrophage uptake of oxLDL. |
| 分子式 |
C15H10O6
|
|
|---|---|---|
| 分子量 |
286.23
|
|
| 精确质量 |
286.047
|
|
| 元素分析 |
C, 62.94; H, 3.52; O, 33.54
|
|
| CAS号 |
520-18-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5280863
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.7±0.1 g/cm3
|
|
| 沸点 |
582.1±50.0 °C at 760 mmHg
|
|
| 熔点 |
276°C
|
|
| 闪点 |
226.1±23.6 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.785
|
|
| LogP |
2.05
|
|
| tPSA |
111.13
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
451
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
OC1=C2C(OC(C3=CC=C(O)C=C3)=C(O)C2=O)=CC(O)=C1
|
|
| InChi Key |
IYRMWMYZSQPJKC-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
|
|
| 化学名 |
3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
|
| 别名 |
3,4',5,7-Tetrahydroxyflavone; Pelargidenolon; Indigo Yellow; Kaempferol; Campherol
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2 mg/mL (6.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2 mg/mL (6.99 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2 mg/mL (6.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5 mg/mL (17.47 mM) in 0.5% CMC/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4937 mL | 17.4685 mL | 34.9369 mL | |
| 5 mM | 0.6987 mL | 3.4937 mL | 6.9874 mL | |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4937 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06060691 | Recruiting | Drug: Kaempferol | Female Sexual Dysfunction | Deraya University | September 20, 2023 | Phase 1 |